filmov
tv
Stoichiometry of a Reaction in Solution

Показать описание
Stoichiometry of a Reaction in Solution
Stoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice Problems
How to Solve Reaction Stoichiometry Problems (Mass-Mass, Mass-Liter, etc.)
Stoichiometry of a Reaction in Solution
Stoichiometry Tutorial: Step by Step Video + review problems explained | Crash Chemistry Academy
Solution Stoichiometry - Finding Molarity, Mass & Volume
Stoichiometry | Mole to mole | Grams to grams | Mole to grams | Grams to mole | Mole ratio
Step by Step Stoichiometry Practice Problems | How to Pass Chemistry
Experiment 4: Stoichiometry of Reactions in Solution
Stoichiometry in chemistry example problem
Stoichiometry Made Easy: Stoichiometry Tutorial Part 1
Stoichiometry Grade 10
Gas Stoichiometry Problems
Stoichiometry - clear & simple (with practice problems) - Chemistry Playlist
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6
Stoichiometry Mole to Mole Conversions - Molar Ratio Practice Problems
Introduction to Limiting Reactant and Excess Reactant
General Chemistry Lab 3 - Stoichiometry of a Precipitation Reaction
Reaction Rates and Stoichiometry- Chemistry Tutorial
Solution Stoichiometry tutorial: How to use Molarity + problems explained | Crash Chemistry Academy
5 1b Reaction Rates and Stoichiometry
What is Stoichiometry?!
Stoichiometry example problem for chemistry: how to calculate the grams of produce produced
Lab Experiment #7: The Stoichiometry of a Chemical Reaction.
Chemistry Lesson: Reaction Stoichiometry
Комментарии
 0:25:16
0:25:16
 0:08:40
0:08:40
 0:10:18
0:10:18
 0:15:24
0:15:24
 0:23:11
0:23:11
 0:17:16
0:17:16
 0:07:09
0:07:09
 0:12:48
0:12:48
 0:00:58
0:00:58
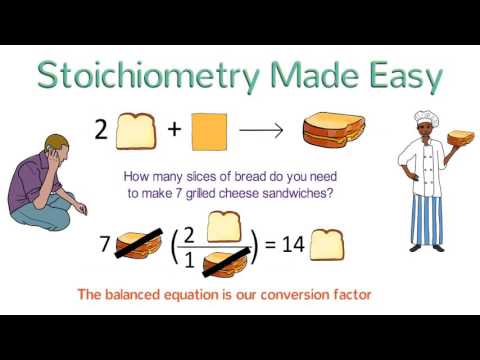 0:06:55
0:06:55
 0:27:18
0:27:18
 0:31:20
0:31:20
 0:26:01
0:26:01
 0:12:47
0:12:47
 0:12:11
0:12:11
 0:16:58
0:16:58
 0:05:44
0:05:44
 0:13:42
0:13:42
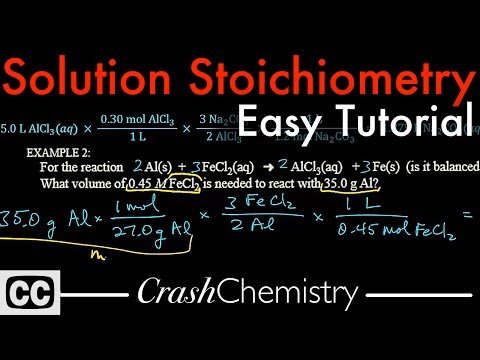 0:10:56
0:10:56
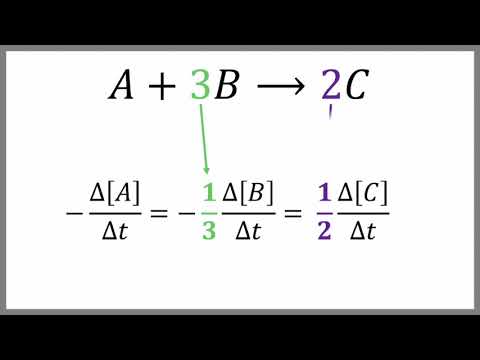 0:05:30
0:05:30
 0:01:05
0:01:05
 0:00:59
0:00:59
 0:14:57
0:14:57
 0:09:17
0:09:17