filmov
tv
\[ \begin{array}{l} \text { Comprehension } \quad \text { For the reaction: } \mathrm{MnBr}_{2}+...

Показать описание
\[
\begin{array}{l}
\text { Comprehension } \quad \text { For the reaction: } \mathrm{MnBr}_{2}+\mathrm{PbO}_{2} \\
+\mathrm{HNO}_{3} \rightarrow \mathrm{HMnO}_{4}+\mathrm{Pb}\left(\mathrm{BrO}_{3}\right)_{2}+\mathrm{Pb}\left(\mathrm{NO}_{3}\right)_{2}+\mathrm{H}_{2} \mathrm{O} \\
\text { (Atomic masses: } \mathbf{M n}=\mathbf{5 5}, \mathbf{B r}=\mathbf{8 0}, \mathbf{P b}=\mathbf{2 0 8})
\end{array}
\]
(Atomic masses: \( \mathrm{Mn}=55, \mathrm{Br}=\mathbf{8 0}, \mathrm{Pb}=\mathbf{2 0 8} \) )
The equivalent weight of \( \mathrm{MnBr}_{2} \) is
(a) 107.5
(b) 215
(c) 12.65
(d) 19.55
\begin{array}{l}
\text { Comprehension } \quad \text { For the reaction: } \mathrm{MnBr}_{2}+\mathrm{PbO}_{2} \\
+\mathrm{HNO}_{3} \rightarrow \mathrm{HMnO}_{4}+\mathrm{Pb}\left(\mathrm{BrO}_{3}\right)_{2}+\mathrm{Pb}\left(\mathrm{NO}_{3}\right)_{2}+\mathrm{H}_{2} \mathrm{O} \\
\text { (Atomic masses: } \mathbf{M n}=\mathbf{5 5}, \mathbf{B r}=\mathbf{8 0}, \mathbf{P b}=\mathbf{2 0 8})
\end{array}
\]
(Atomic masses: \( \mathrm{Mn}=55, \mathrm{Br}=\mathbf{8 0}, \mathrm{Pb}=\mathbf{2 0 8} \) )
The equivalent weight of \( \mathrm{MnBr}_{2} \) is
(a) 107.5
(b) 215
(c) 12.65
(d) 19.55
 0:09:25
0:09:25
 0:09:43
0:09:43
 0:14:15
0:14:15
 0:10:51
0:10:51
 0:02:12
0:02:12
 0:04:16
0:04:16
 0:04:21
0:04:21
 0:13:40
0:13:40
 0:08:27
0:08:27
 0:07:55
0:07:55
 0:05:13
0:05:13
 0:02:44
0:02:44
 0:02:39
0:02:39
 0:02:24
0:02:24
 0:10:20
0:10:20
 0:13:24
0:13:24
 0:12:00
0:12:00
 0:09:59
0:09:59
 0:02:58
0:02:58
 0:11:33
0:11:33
 0:02:27
0:02:27
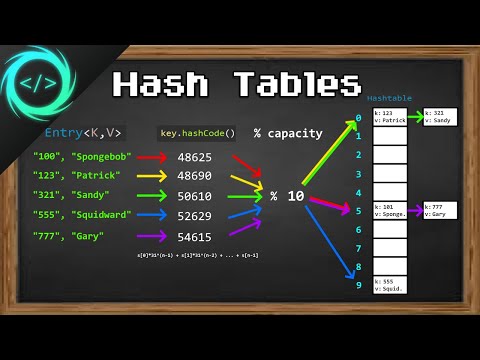 0:13:26
0:13:26
 0:01:41
0:01:41
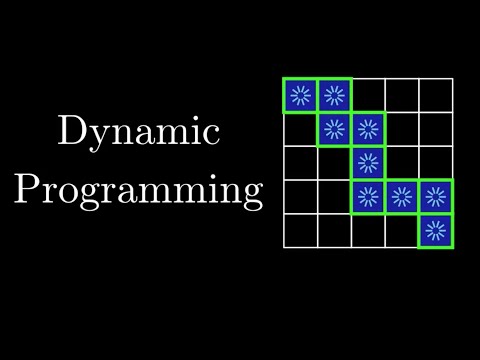 0:21:27
0:21:27