filmov
tv
Atom animated--nucleus & electron orbitals (electron cloud).

Показать описание
This animation of the atom starts with a solid little black ball, representing the nucleus. Of course, the nucleus is not a hard little ball. For one thing, it has components—proton(s) and, for most elements, neutrons. These components are subatomic and have properties of both waves and particles. But for simplicity, the nucleus is shown as if it were a little ball of matter.
The nucleus is positively charged due to the presence of protons. Each proton has one unit of positive charge. Neutrons have no charge. The positive charge around the nucleus is shown as a kind of scaffolding or network around it.
The positive nucleus attracts electrons. Each electron has one unit of negative charge. If the nucleus has only one proton, it will attract one electron, and the two will balance each other perfectly. The positive and negative charges cancel to make the atom electrically neutral.
The animation first shows the nucleus attracting one electron. One proton and one electron is the simplest atom, hydrogen. The animation notes this with the symbol H.
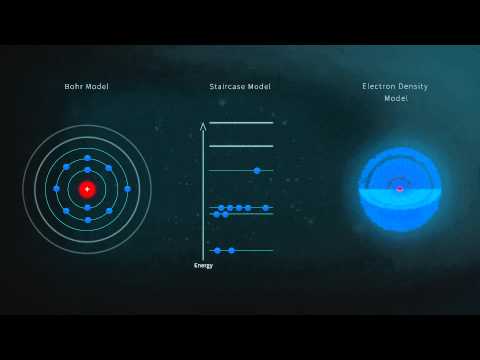
The electron is shown as a spherical electron cloud or “orbital.” Contrary to what is often taught in school, the electron is not orbiting the nucleus like the Earth orbits the sun. That visualization is easy to get across in school but is almost a century out-of-date. Today, the electron is thought of as occupying a region around the nucleus called an “orbital.”
The electron orbital within the hydrogen atom can have a number of different shapes. Orbital shape depends upon how energized the electron is. However, this animation keeps it simple by showing only one shape, a sphere.
In the animation, electrons are added to the atom, one by one. This would not occur in nature—electrons do not simply jump into hydrogen atoms to make other elements. The animation is showing a series of other elements, not how they’re created.
Elements with more electrons must also have more protons in the nucleus to keep the charge of the atom balanced. Neutrons are also needed to allow the nucleus to hold together. A balance of protons and electrons, with the help of neutrons, results in atoms that are electrically neutral. The animation, again, simplifies by not showing us the contents of the nucleus.
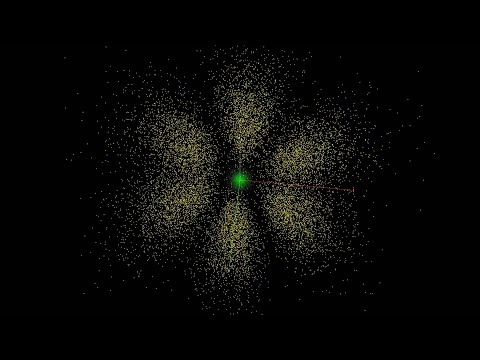
The other elements shown with spherical orbitals are helium, symbolized He; lithium, symbolized Li; and beryllium, symbolized Be. The orbital is shown as larger for the atoms with more electrons. As in the case of hydrogen, at different energy levels, the electron orbitals can take on other shapes besides spherical. And, while this is not indicated, each electron has its own orbital. So, the atom is actually a nucleus with a set of orbitals around it, one orbital for each electron.
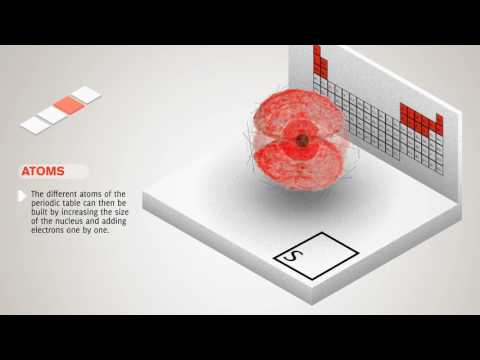
The animation changes to show a dumbbell-shaped orbital for boron, symbolized B, and carbon, symbolized C. More complex orbital shapes are shown for other elements: nitrogen, N; oxygen, O; and fluorine, F, and so on.
While the animation has selected one set of orbital shapes for each of the elements shown, in reality, each element can have many different sets of orbital shapes depending on energy levels of its electrons.
Комментарии
 0:01:04
0:01:04
 0:05:35
0:05:35
 0:05:50
0:05:50
 0:01:54
0:01:54
 0:08:39
0:08:39
 0:02:02
0:02:02
 0:06:25
0:06:25
 0:00:24
0:00:24
 0:02:50
0:02:50
 0:00:48
0:00:48
 0:05:41
0:05:41
 0:00:07
0:00:07
 0:02:43
0:02:43
 0:00:07
0:00:07
 0:04:02
0:04:02
 0:05:23
0:05:23
 0:04:52
0:04:52
 0:14:28
0:14:28
 0:01:52
0:01:52
 0:01:00
0:01:00
 0:21:59
0:21:59
 0:06:31
0:06:31
 0:04:58
0:04:58
 0:02:32
0:02:32