filmov
tv
Wave Model of an Electron

Показать описание
135 - Wave Model of an Electron
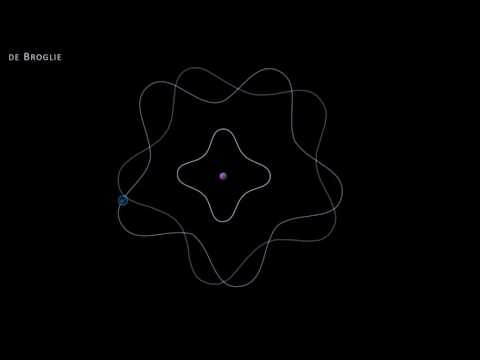
The wave model of the electron can be used to explain the Bohr model. Electrons are found in certain orbits because they interfere with themselves and create standing waves. When the wavelengths don’t match up with a whole integer they will create destructive interference. When electrons fall to a lower orbit they emit a photon. They can move to a higher orbit by absorbing an identical photon.
Do you speak another language? Help me translate my videos:
Music Attribution
Title: String Theory
Artist: Herman Jolly
All of the images are licensed under creative commons and public domain licensing:
The wave model of the electron can be used to explain the Bohr model. Electrons are found in certain orbits because they interfere with themselves and create standing waves. When the wavelengths don’t match up with a whole integer they will create destructive interference. When electrons fall to a lower orbit they emit a photon. They can move to a higher orbit by absorbing an identical photon.
Do you speak another language? Help me translate my videos:
Music Attribution
Title: String Theory
Artist: Herman Jolly
All of the images are licensed under creative commons and public domain licensing:
Wave Model of an Electron
Quantum Physics for Dummies - Is Electron a Wave or a Particle?
Louis de Broglie's explanation of Bohr's atomic model
demonstrating de Broglie's hypothesis of electrons as standing waves
A Better Way To Picture Atoms
What is the Wave/Particle Duality? Part 1
Electron Wave in Bohr Model of Atom!! (Quantization)
Quantum Mechanics of the Electron
APEC 11/23: Fine-Structure Constant, Bubble Fusion & Warp-Drives
What Does An Electron Look Like
The Quantum Mechanical model of an atom. What do atoms look like? Why?
Wave-Particle Duality and the Photoelectric Effect
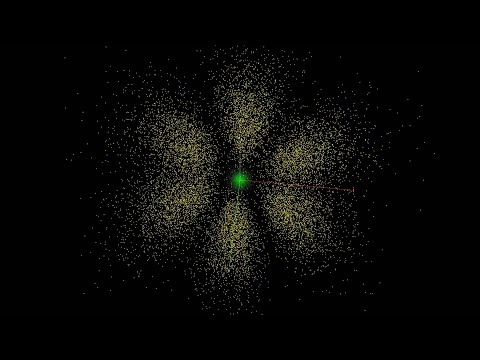
Atomic orbitals 3D
Quantum Numbers, Atomic Orbitals, and Electron Configurations
Quantum Mechanics and the Schrödinger Equation
The Double-Slit Experiment
Explanation of Wave Nature of Electron
Bohr Model of the Hydrogen Atom
Electron is Wave or Particle : Nobel Prize,JJ Thomson & GP Thomson
Is light a particle or a wave? - Colm Kelleher
Orbitals: Crash Course Chemistry #25
Electrons act like waves, peobabilities of an orbital #generalknowledge #electron #atom #nucleus
Wave Nature of Electron
What Does an Electron Look Like?
Комментарии
 0:04:02
0:04:02
 0:06:05
0:06:05
 0:08:12
0:08:12
 0:09:34
0:09:34
 0:05:35
0:05:35
 0:01:07
0:01:07
 0:13:47
0:13:47
 0:04:01
0:04:01
 4:26:05
4:26:05
 0:01:00
0:01:00
 0:14:26
0:14:26
 0:03:56
0:03:56
 0:05:50
0:05:50
 0:08:42
0:08:42
 0:06:28
0:06:28
 0:04:23
0:04:23
 0:00:10
0:00:10
 0:04:50
0:04:50
 0:00:23
0:00:23
 0:04:24
0:04:24
 0:10:52
0:10:52
 0:00:30
0:00:30
 0:00:07
0:00:07
 0:06:31
0:06:31