filmov
tv
Year 1 Organic Chemistry Mechanisms Explained | Ultimate AS & A-Level Chemistry Guide

Показать описание
Year 1 Organic Chemistry Mechanisms Explained | Ultimate AS & A-Level Chemistry Guide
Welcome to The Chemistry Tutor! In this video, we’re diving into the essential Year 1 organic chemistry mechanisms that every AS & A-Level student needs to master. This ultimate guide covers key reactions and concepts to help you excel in your studies and exams.
What You’ll Learn in This Video:
What are Mechanisms For? – Understand the purpose and importance of chemical mechanisms.
Electrophilic Addition Explained – Get a clear breakdown of this fundamental reaction.
Bromine as an Electrophile – Learn how bromine functions in electrophilic addition.
Unsymmetrical Alkenes – Explore the behavior and reactions of unsymmetrical alkenes.
Carbocation Stability – Discover how carbocation stability affects reactions.
Nucleophilic Substitution – Dive into this key mechanism and its applications.
Ammonia as a Nucleophile – Understand how ammonia acts in nucleophilic substitution.
Elimination Reactions – Learn the details of elimination mechanisms and their outcomes.
Mixtures of Alkene Products – Explore how elimination can lead to multiple alkene products.
Elimination vs Substitution – Compare and contrast these important reaction types.
Elimination from Alcohols – Examine the process and implications of elimination reactions involving alcohols.
Isomeric Alkenes – Understand the formation and significance of isomeric alkenes.
By the end of this video, you'll have a solid grasp of these fundamental mechanisms and be better prepared for your AS & A-Level Chemistry exams. If you find this guide helpful, don’t forget to like, subscribe, and share! Drop your questions in the comments, and join our community of chemistry learners.
Explore More:
• Year 2 organic mechanisms video:
Stay ahead of your studies and hit the bell 🔔 for more AS & A-Level Chemistry tutorials!
Connect with Us:
• Instagram: @chemistrytutor123
• TikTok: @chemistrytutor123
Join our community and take your AS & A-Level Chemistry knowledge to the next level!
#ASLevelChemistry #OrganicMechanisms #ElectrophilicAddition #NucleophilicSubstitution #EliminationReactions #ChemistryRevision
00:00 Introduction & Overview – What to Expect in This Video
00:30 Purpose of Mechanisms – Why They Matter in Chemistry
02:03 Electrophilic Addition Explained – Key Concepts and Reactions
06:30 Role of Bromine as an Electrophile – Detailed Analysis
08:40 Reactions of Unsymmetrical Alkenes – How They Differ
12:28 Understanding Carbocation Stability – Essential for Reactions
13:56 Nucleophilic Substitution Explained – Mechanism and Examples
17:06 Ammonia as a Nucleophile – Its Role in Substitution Reactions
22:10 Elimination Reactions – Mechanisms and Key Points
25:10 Products of Elimination Reactions – Understanding Alkene Mixtures
27:06 Comparing Elimination vs Substitution – Which Reaction to Expect
29:13 Elimination from Alcohols – Process and Mechanism Explained
31:38 Isomeric Alkenes
Welcome to The Chemistry Tutor! In this video, we’re diving into the essential Year 1 organic chemistry mechanisms that every AS & A-Level student needs to master. This ultimate guide covers key reactions and concepts to help you excel in your studies and exams.
What You’ll Learn in This Video:
What are Mechanisms For? – Understand the purpose and importance of chemical mechanisms.
Electrophilic Addition Explained – Get a clear breakdown of this fundamental reaction.
Bromine as an Electrophile – Learn how bromine functions in electrophilic addition.
Unsymmetrical Alkenes – Explore the behavior and reactions of unsymmetrical alkenes.
Carbocation Stability – Discover how carbocation stability affects reactions.
Nucleophilic Substitution – Dive into this key mechanism and its applications.
Ammonia as a Nucleophile – Understand how ammonia acts in nucleophilic substitution.
Elimination Reactions – Learn the details of elimination mechanisms and their outcomes.
Mixtures of Alkene Products – Explore how elimination can lead to multiple alkene products.
Elimination vs Substitution – Compare and contrast these important reaction types.
Elimination from Alcohols – Examine the process and implications of elimination reactions involving alcohols.
Isomeric Alkenes – Understand the formation and significance of isomeric alkenes.
By the end of this video, you'll have a solid grasp of these fundamental mechanisms and be better prepared for your AS & A-Level Chemistry exams. If you find this guide helpful, don’t forget to like, subscribe, and share! Drop your questions in the comments, and join our community of chemistry learners.
Explore More:
• Year 2 organic mechanisms video:
Stay ahead of your studies and hit the bell 🔔 for more AS & A-Level Chemistry tutorials!
Connect with Us:
• Instagram: @chemistrytutor123
• TikTok: @chemistrytutor123
Join our community and take your AS & A-Level Chemistry knowledge to the next level!
#ASLevelChemistry #OrganicMechanisms #ElectrophilicAddition #NucleophilicSubstitution #EliminationReactions #ChemistryRevision
00:00 Introduction & Overview – What to Expect in This Video
00:30 Purpose of Mechanisms – Why They Matter in Chemistry
02:03 Electrophilic Addition Explained – Key Concepts and Reactions
06:30 Role of Bromine as an Electrophile – Detailed Analysis
08:40 Reactions of Unsymmetrical Alkenes – How They Differ
12:28 Understanding Carbocation Stability – Essential for Reactions
13:56 Nucleophilic Substitution Explained – Mechanism and Examples
17:06 Ammonia as a Nucleophile – Its Role in Substitution Reactions
22:10 Elimination Reactions – Mechanisms and Key Points
25:10 Products of Elimination Reactions – Understanding Alkene Mixtures
27:06 Comparing Elimination vs Substitution – Which Reaction to Expect
29:13 Elimination from Alcohols – Process and Mechanism Explained
31:38 Isomeric Alkenes
Комментарии
 0:33:01
0:33:01
 0:34:45
0:34:45
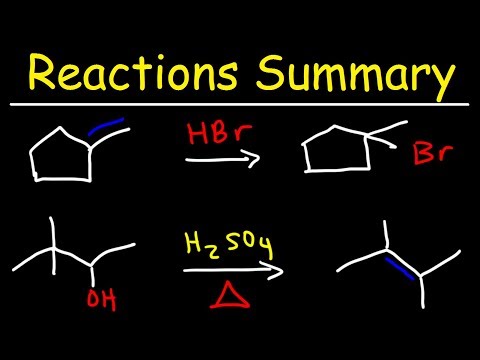 0:38:34
0:38:34
 0:12:43
0:12:43
 0:43:18
0:43:18
 0:13:02
0:13:02
 0:07:13
0:07:13
 0:07:40
0:07:40
 0:15:15
0:15:15
 0:13:15
0:13:15
 0:18:52
0:18:52
 0:26:37
0:26:37
 0:42:23
0:42:23
 0:11:22
0:11:22
 0:02:25
0:02:25
 0:13:52
0:13:52
 0:07:29
0:07:29
 0:12:19
0:12:19
 0:16:54
0:16:54
 0:05:30
0:05:30
 0:13:07
0:13:07
 0:49:33
0:49:33
 0:53:35
0:53:35
 1:10:07
1:10:07