filmov
tv
Violations to Aufbau principle, Hund's rule and the Pauli exclusion principle.

Показать описание
Aufbau Principle: Electrons fill the lower energy orbitals first.
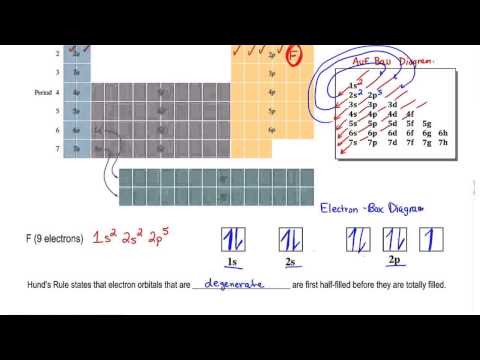
Hund's Rule: Orbitals must be ½ filled before pairing(for the same shell, s,p,d etc.).
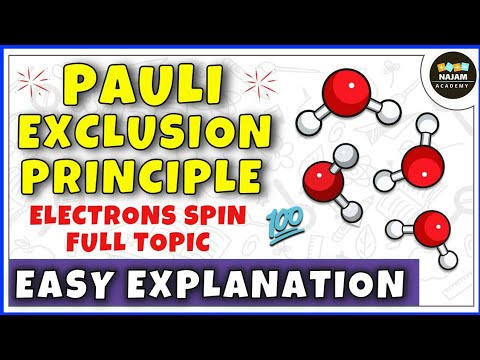
Pauli Exclusion Principle: Electrons in the same orbital must have opposite spin.
Hund's Rule: Orbitals must be ½ filled before pairing(for the same shell, s,p,d etc.).
Pauli Exclusion Principle: Electrons in the same orbital must have opposite spin.
Aufbau's Principle, Hund's Rule & Pauli's Exclusion Principle - Electron Configur...
Violations to Aufbau principle, Hund's rule and the Pauli exclusion principle.
Aufbau Principle, Pauli Exclusion Principle, Hund's Rule EXPLAINED With Violations!
Aufbau Principle, Hund's Rule, Pauli Exclusion Principle Explained in Four Minutes w/ Examples
Pauli Principle, Hund's Rule, Octet Rule & Aufbau Principle
Aufbau Pauli and Hund violations 1 5
CONFUSION: Aufbau's vs Hund's vs Pauli exclusion🔥| Neet 2022 | Last Moment Tricks
Aufbau , Hund and pauli's rule in 1 min🔥 #neet #shorts #viral
Hund's Rule | Chemistry
Aufbau Principle || Atomic Structure 06 || Classs 11 chap 2|| Rules for Filling Of electrons || IIT
Aufbau Principle | Chemistry
How To Understand Aufbau Principle, Pauli Exclusion Principle, And Hund’s Rule In Chemistry
Aufbau principle|| Which is rule is violated in the configuration || class10
How electrons adjust in orbitals-Hunds rule, Pauli exclusion principal and Aufbau principal
SAMPLE PROBLEM: Exceptions to the Aufbau Principle
3 Confusing Rules- Aufbau,Hund,Pauli exclusion | Super Easy Tricks🔥🔥 | Neet 2021 | NEET-IITJEE
The orbital diagram in which the Aufbau principle is violated is | 11 | STRUCTURE OF ATOM | CHE...
Hund's Rule
Electron Configuration - Pauli Exclusion Principle, Aufbau Principle and Hund's Rule Explained.
Pauli Exclusion principle - Hund's rule - Aufbau principle: How electrons occupy orbitals
Best explanation of Hund's Rule😂 - You will never forget #shorts #youtubeshorts #science
4.3 Aufbau Principle, Hund's Rule & Pauli Exclusion Principle
Aufbau principal Pauli exclusion principle, hund's rule | Simplified
Pauli Exclusion Principle
Комментарии
 0:05:24
0:05:24
 0:04:27
0:04:27
 0:03:59
0:03:59
 0:03:54
0:03:54
 0:10:47
0:10:47
 0:12:13
0:12:13
 0:03:56
0:03:56
 0:01:00
0:01:00
 0:08:07
0:08:07
 0:09:56
0:09:56
 0:06:54
0:06:54
 0:03:36
0:03:36
 0:08:06
0:08:06
 0:00:16
0:00:16
 0:09:46
0:09:46
 0:01:50
0:01:50
 0:02:11
0:02:11
 0:02:39
0:02:39
 0:05:13
0:05:13
 0:15:25
0:15:25
 0:00:43
0:00:43
 0:05:40
0:05:40
 0:04:21
0:04:21
 0:08:23
0:08:23