filmov
tv
Alkene Addition Reactions Made Easy! - Product Prediction Tips! - Organic Chemistry

Показать описание
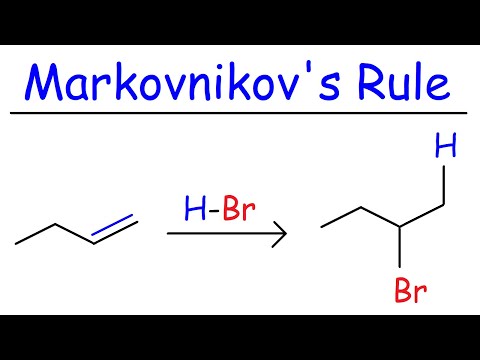
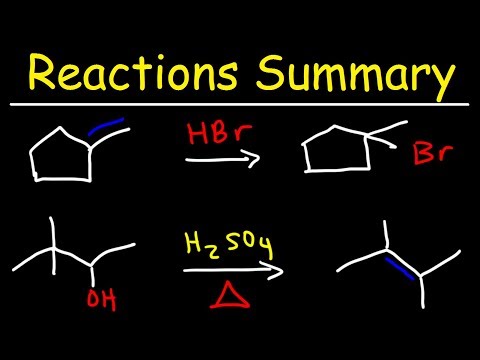
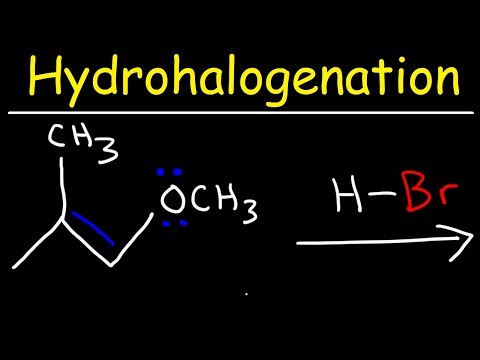
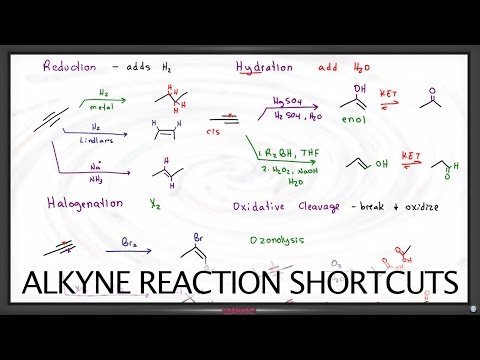
This video is mean't to just help you guys out with Product Prediction in case you're stuck and you only have a few minutes left and have nothing to lose and no time to go through the mechanisms to make sure your answer is right. In this video I show the usual pattern that Hydrohalogenation, Halogenation, Hydroboration, Acid Catalyzed Hydration, Acidy Catalyzed Alkoxy Addition, and Halohydrin formation occurs.
*******2020 Mid CoVid Update*******
Hey guys! So YouTube removed all annotation bubbles that used to pop up on videos. So some of my corrections don’t show up anymore. I think the main correction that’s missing is that Hydration via Hydroboration Oxidation causes the H to go on the “Good” more substituted carbon of the alkene and the OH to go onto the “Bad/Crappy” (less substituted carbon).
But both must be double WEDGED or double DASHED (unless of course the carbon they are sitting on isn’t a Chiral Center where you don’t need to show stereochemistry aka wedging/dashing. Some profs will teach that the H and the OH will only yield a major product where they are on the “Less Hindered Face” of the alkene. Meaning if you have an alkene inside a 5 member ring (cyclopentane) and there is already a wedged (pointing upwards) methyl group near the alkene, the H and the OH would add onto the alkene carbons with both being dashed to avoid crowding the top face of the molecule (where the wedged methyl already is.)
●Private Tutoring Information:
Make sure you share this with your friends if you found it helpful, and I would love it if you leave some comments to let me know if I'm on the right track ;).
Subscribe to get updated when I make new videos!
Connect with me on Facebook/Instagram/Twitter!
*******2020 Mid CoVid Update*******
Hey guys! So YouTube removed all annotation bubbles that used to pop up on videos. So some of my corrections don’t show up anymore. I think the main correction that’s missing is that Hydration via Hydroboration Oxidation causes the H to go on the “Good” more substituted carbon of the alkene and the OH to go onto the “Bad/Crappy” (less substituted carbon).
But both must be double WEDGED or double DASHED (unless of course the carbon they are sitting on isn’t a Chiral Center where you don’t need to show stereochemistry aka wedging/dashing. Some profs will teach that the H and the OH will only yield a major product where they are on the “Less Hindered Face” of the alkene. Meaning if you have an alkene inside a 5 member ring (cyclopentane) and there is already a wedged (pointing upwards) methyl group near the alkene, the H and the OH would add onto the alkene carbons with both being dashed to avoid crowding the top face of the molecule (where the wedged methyl already is.)
●Private Tutoring Information:
Make sure you share this with your friends if you found it helpful, and I would love it if you leave some comments to let me know if I'm on the right track ;).
Subscribe to get updated when I make new videos!
Connect with me on Facebook/Instagram/Twitter!
Комментарии
 0:08:52
0:08:52
 0:12:53
0:12:53
 0:05:45
0:05:45
 1:10:23
1:10:23
 0:15:30
0:15:30
 0:06:39
0:06:39
 0:11:17
0:11:17
 0:03:25
0:03:25
 0:18:10
0:18:10
 0:34:45
0:34:45
 0:00:06
0:00:06
 0:08:37
0:08:37
 0:38:34
0:38:34
 0:12:19
0:12:19
 0:00:38
0:00:38
 0:04:23
0:04:23
 0:19:56
0:19:56
 0:18:32
0:18:32
 0:11:01
0:11:01
 0:01:00
0:01:00
 0:06:15
0:06:15
 0:00:32
0:00:32
 0:03:28
0:03:28
 0:21:34
0:21:34