filmov
tv
Hydrohalogenation - Alkene Reaction Mechanism

Показать описание
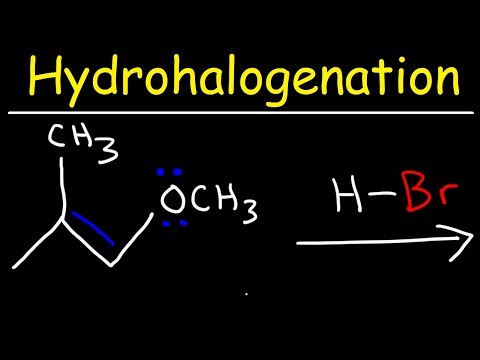
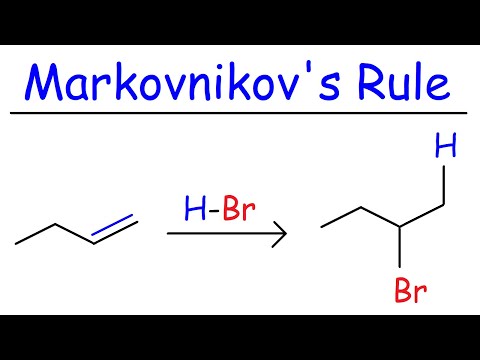
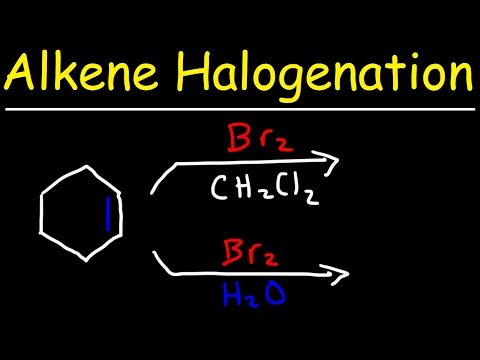
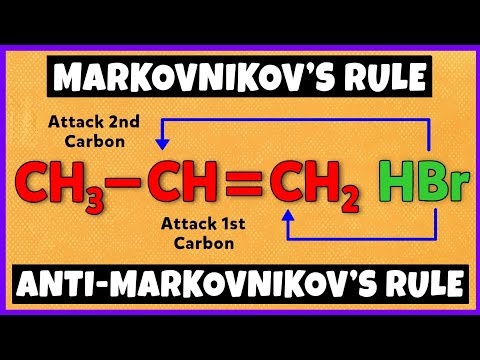
This organic chemistry video tutorial provides a basic introduction into hydrohalogenation of alkenes. It provides the reaction mechanism as well. Examples include carbocation rearrangements such as hydride shifts, methyl shifts, and the stereochemistry of the products are mentioned as well.
Alkene Reaction - Stereochemistry Test Question:
Simmons Smith Reaction Test Question:
Syn Vs Anti Addition Test Question:
Alkene Epoxidation Test Question:
Alkoxymercuration Demercuration Test Question:
Ozonolysis Test Question:
________________________________
Stereochemistry R/S Configuration:
Optical Activity & Specific Rotation:
SN1, SN2, E1, E2 Reaction Mechanisms:
SN1, SN2, E1, E2 - Practice Test:
Alkene Reactions Review:
________________________________
Alkyne Reactions Review:
Organic Chemistry PDF Worksheets:
Organic Chemistry 1 Exam 2 Playlist:
Organic Chemistry 1 Final Exam Review:
Full-Length Videos and Worksheets:
Alkene Reaction - Stereochemistry Test Question:
Simmons Smith Reaction Test Question:
Syn Vs Anti Addition Test Question:
Alkene Epoxidation Test Question:
Alkoxymercuration Demercuration Test Question:
Ozonolysis Test Question:
________________________________
Stereochemistry R/S Configuration:
Optical Activity & Specific Rotation:
SN1, SN2, E1, E2 Reaction Mechanisms:
SN1, SN2, E1, E2 - Practice Test:
Alkene Reactions Review:
________________________________
Alkyne Reactions Review:
Organic Chemistry PDF Worksheets:
Organic Chemistry 1 Exam 2 Playlist:
Organic Chemistry 1 Final Exam Review:
Full-Length Videos and Worksheets:
Комментарии
 0:12:19
0:12:19
 0:04:19
0:04:19
 0:10:04
0:10:04
 0:04:55
0:04:55
 0:11:17
0:11:17
 0:03:07
0:03:07
 0:09:48
0:09:48
 0:10:25
0:10:25
 0:36:03
0:36:03
 0:19:06
0:19:06
 0:00:06
0:00:06
 0:12:53
0:12:53
 0:18:32
0:18:32
 0:20:38
0:20:38
 0:05:45
0:05:45
 1:10:23
1:10:23
 0:00:38
0:00:38
 0:24:35
0:24:35
 0:14:10
0:14:10
 0:13:13
0:13:13
 0:12:48
0:12:48
 0:04:35
0:04:35
 0:36:18
0:36:18
 0:04:54
0:04:54