filmov
tv
Alkene Reactions #1 - Narrated Answer Key

Показать описание
This is a predict-the-product problem set on alkene and alkene reactions (addition and reduction reactions).
Alkene Reactions #1 - Narrated Answer Key
Alkene Reactions
Alkene Reactions 1: Markovnikov Addition & Rearrangement
Alkene reactions - part I
Alkene Addition Reactions: Crash Course Organic Chemistry #16
Alkene Reactions 1: Simple Guide For Predicting The Products And Stereochemistry | Orgo With Mayya
8.1 Introduction to Alkene Addition Reactions; Markovnikov's Rule and Syn vs Anti | OChemistry
Alkene reactions
GCSE Chemistry - Addition Reactions of Alkenes #55
All Alkene Reactions in 4 minutes!
Reactions of Alkenes--Tackling ALL of Them (Worksheet Solutions Walkthrough)
Anti Addition vs Syn Addition - Alkene Reactions
Quick Organic Chemistry 1 Reactions Review - Alkene Alkyne Radical Substitution Elimination
Alkene Reactions: Intro
Alkene, Alkyne Reactions || Orgo Made Easy
Quick Revision - Alkenes (reactions)
Alkene and Alkyne reactions homework
Alkene Reactions (Electrophilic Addition Mechanism)
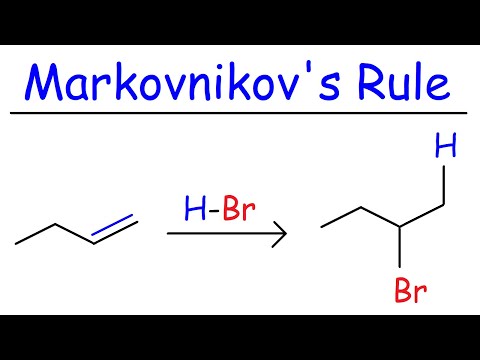
Markovnikov's Rule
8 - Reactions of Alkenes
5 Alkene Reactions
Alkene reactions worked example II
8.8 How to Predict the Products of Alkene Addition Reactions | Organic Chemistry
Reactions of Alkanes, Alkenes and Alkynes with Examples
Комментарии
 0:19:56
0:19:56
 1:10:23
1:10:23
 0:18:35
0:18:35
 0:12:10
0:12:10
 0:12:53
0:12:53
 0:14:04
0:14:04
 0:08:37
0:08:37
 0:00:06
0:00:06
 0:05:45
0:05:45
 0:04:23
0:04:23
 0:39:29
0:39:29
 0:06:15
0:06:15
 0:16:54
0:16:54
 0:22:34
0:22:34
 0:01:00
0:01:00
 0:05:35
0:05:35
 0:14:13
0:14:13
 0:20:08
0:20:08
 0:11:17
0:11:17
 1:21:48
1:21:48
 0:00:59
0:00:59
 0:09:19
0:09:19
 0:06:39
0:06:39
 0:25:53
0:25:53