filmov
tv
Ionic and Covalent Bonding

Показать описание
This video explains all about Ionic and Covalent bonding :) enjoy!
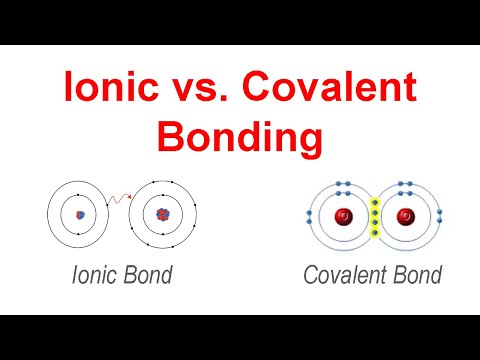
Chemical Bonding - Ionic vs. Covalent Bonds
The Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar
Chemical Bonds: Ionic and Covalent
GCSE Chemistry - What is Ionic Bonding? How Does Ionic Bonding Work? Ionic Bonds Explained #14
Ionic and Covalent Bonding - Chemistry
Ionic vs Covalent Bonds
Ionic and Covalent Bonds | Chemical Bonding
Ionic and Covalent Bonding | Chemical Bonding
Class 11 Chemistry Chapter 4 | Chemical Bonding - Coordination Bond | L-46 | Akshay Sir
How atoms bond - George Zaidan and Charles Morton
Types of Bonding (Ionic, Covalent, Metallic) - GCSE Chemistry Revision
GCSE Chemistry - Covalent Bonding #16
Chemical Bonding Explained | Ionic, Covalent and Metallic | GCSE Chemistry
Atomic Hook-Ups - Types of Chemical Bonds: Crash Course Chemistry #22
Ionic Bonding Introduction
Covalent Compounds VS Ionic Compounds
Ionic and Covalent Bonds, Hydrogen Bonds, van der Waals - 4 types of Chemical Bonds in Biology
Science Raps: GCSE Chemistry - Ionic and Covalent Bonding
Ionic Bonds, Polar Covalent Bonds, and Nonpolar Covalent Bonds
Types Of Chemical Bonds - What Are Chemical Bonds - Covalent Bonds And Ionic Bonds - What Are Ions
Chemical Formulas, Ionic & Covalent Bonds in Chemistry - [1-2-14]
What are Ionic Bonds? | Properties of Matter | Chemistry | FuseSchool
Ionic vs Covalent Bonds (CLEAN RADIO EDIT)
How to tell if Ionic Bond or Covalent Bond
Комментарии
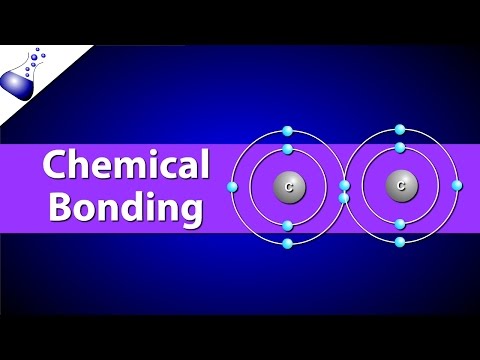 0:02:15
0:02:15
 0:03:33
0:03:33
 0:04:30
0:04:30
 0:04:12
0:04:12
 0:21:57
0:21:57
 0:03:21
0:03:21
 0:07:04
0:07:04
 0:47:18
0:47:18
 0:23:10
0:23:10
 0:03:34
0:03:34
 0:11:50
0:11:50
 0:05:33
0:05:33
 0:03:03
0:03:03
 0:09:46
0:09:46
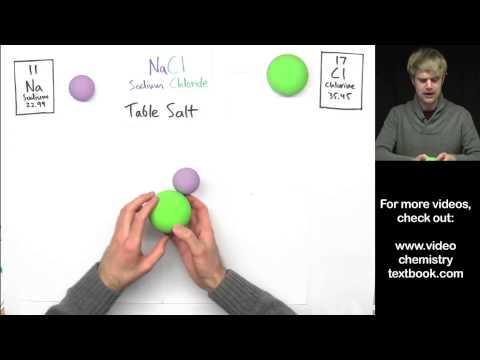 0:07:20
0:07:20
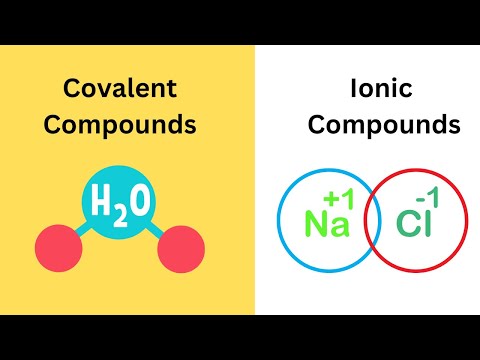 0:02:55
0:02:55
 0:08:50
0:08:50
 0:01:03
0:01:03
 0:11:00
0:11:00
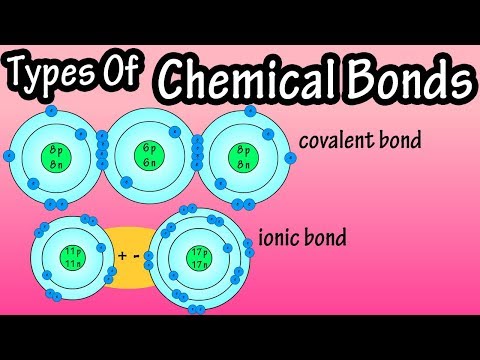 0:04:18
0:04:18
 0:59:54
0:59:54
 0:02:55
0:02:55
 0:01:27
0:01:27
 0:04:53
0:04:53