filmov
tv
Calculate Average Kinetic Energy, Total Transitional Kinetic Energy, RMS Speed of Atoms

Показать описание
Follow us:
Q1. Two moles of helium are in a tank at 25°C. Find:
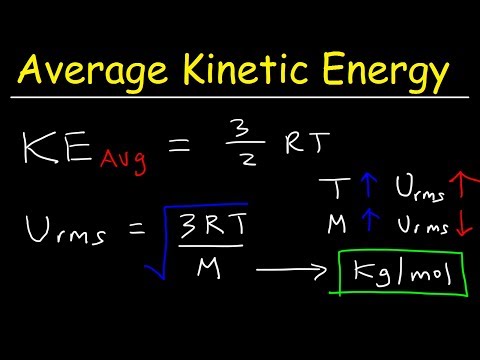
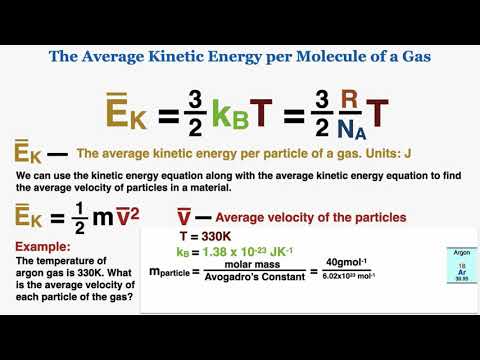
a) the average kinetic energy per molecule
b) the total translational kinetic energy
c) the root-mean-square (rms) speed of the atoms
Q2. Isotopes of uranium are sometimes separated by gaseous diffusion. The lighter isotope, having a higher speed, diffuses more rapidly than the heavier isotope. What is the ratio of the root-mean-square (rms) speeds of U-235 and U-238?
Thermodynamics is a very neat and compact subject in that the equation of state pV = nRT relation can be derived with some simple assumptions and the application of basic force, momentum, and energy concepts. The kinetic theory of gases leads directly to the equation of state for a gas.
Start with N identical molecules in a cube of volume V and sides d, and make the following assumptions and restrictions on their behavior:
1. The molecules are small compared with the dimensions of the container and the distance between molecules.
2. Only collisions with the walls are considered; collisions between molecules are ignored.
3. Collisions with the walls are elastic, and the momentum transferred is manifest as pressure on the walls.
4. The molecules and the walls are in thermal equilibrium. There is no energy transfer between the molecules and the walls.
 0:07:11
0:07:11
 0:02:34
0:02:34
 0:06:47
0:06:47
 0:12:51
0:12:51
 0:02:34
0:02:34
 0:29:23
0:29:23
 0:02:10
0:02:10
 0:03:27
0:03:27
 2:07:36
2:07:36
 0:05:38
0:05:38
 0:03:28
0:03:28
 0:01:46
0:01:46
 0:00:11
0:00:11
 0:00:16
0:00:16
 0:13:18
0:13:18
 0:03:28
0:03:28
 0:04:13
0:04:13
 0:08:12
0:08:12
 0:03:23
0:03:23
 0:00:28
0:00:28
 0:06:45
0:06:45
 0:00:33
0:00:33
 0:02:40
0:02:40
 0:01:13
0:01:13