filmov
tv
Example: Evaluating work in an ideal gas Carnot cycle

Показать описание
5 Self Evaluation Questions To Tackle In Your Next Self Assessment At Work
Self Evaluation | Performance Review Tips to Slay Your Self Assessment At Work
How To Prepare A Self-Evaluation - Business English Tips
Example: Evaluating work in an ideal gas Carnot cycle
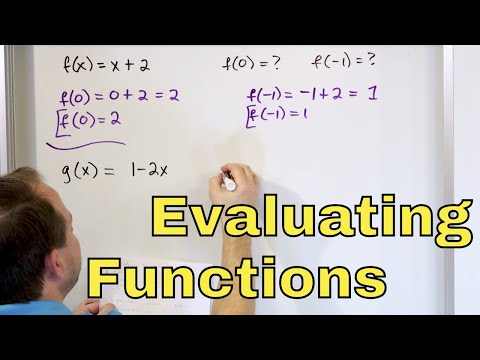
Learn how to evaluate for a function
Dr. Karen Yarrish - Poor Performance Evaluation
What is monitoring and evaluation? #monitoringandevaluation #motivation #evaluation
07 - Evaluating Functions in Algebra, Part 1 (Function Notation f(x), Examples & Definition)
Essay Writing Course Lesson 08: Evaluation Essay
What is Job Evaluation ?
What Happens During a Psychological Evaluation
Program Evaluation Example
What is program evaluation?: A Brief Introduction
Evaluation and Assessment
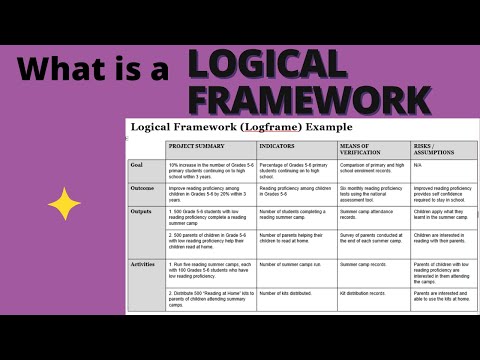
How to develop a Monitoring and Evaluation Framework | M&E Daily with COACH ALEXANDER
ASSESSMENT versus EVALUATION
How to develop a logical framework #logicalframework #monitoringandevaluation #evaluation #m&e
Evaluating Piecewise Functions | PreCalculus
How to Evaluate Employee Performance Effectively | Ideas to keep you ahead in the business
How to Evaluate a Teacher’s Performance
Intro to Evaluating Algebraic Expressions | How to Evaluate Algebraic Expressions | Math with Mr. J
Appraisal Meeting Tips For Employee | Performance Review Meeting With Manager | Simplilearn
How Library Stuff Works: How to Evaluate Resources (the CRAAP Test)
Employee Performance Evaluation | Employee Assessment Software | Task Based Appraisal
Комментарии
 0:02:12
0:02:12
 0:09:43
0:09:43
 0:15:10
0:15:10
 0:08:31
0:08:31
 0:02:15
0:02:15
 0:03:50
0:03:50
 0:03:11
0:03:11
 0:09:53
0:09:53
 0:10:13
0:10:13
 0:01:02
0:01:02
 0:01:31
0:01:31
 0:03:24
0:03:24
 0:05:38
0:05:38
 0:08:11
0:08:11
 0:15:49
0:15:49
 0:02:40
0:02:40
 0:03:36
0:03:36
 0:05:47
0:05:47
 0:01:54
0:01:54
 0:06:43
0:06:43
 0:05:32
0:05:32
 0:02:43
0:02:43
 0:02:10
0:02:10
 0:01:01
0:01:01