filmov
tv
How Collision Theory Relates to Equilibrium // HSC Chemistry

Показать описание
This video is about Module 5 Lesson 2 – Collision Theory and Equilibrium of the HSC Chemistry syllabus.
Syllabus Dotpoints
*Investigate the relationship between collision theory and reaction rate in order to analyse chemical equilibrium reactions (ACSCH070, ACSCH094)
Time Stamp
07:17 Equilibrium Disturbance
07:45 How Concentration Affects Equilibrium
09:30 How Pressure/Volume Affects Equilibrium
10:54 How Temperature Affects Equilibrium
12:28 Microscopic vs Macroscopic Changes at Equilibrium
14:01 How Catalyst Affects Equilibrium
Syllabus Dotpoints
*Investigate the relationship between collision theory and reaction rate in order to analyse chemical equilibrium reactions (ACSCH070, ACSCH094)
Time Stamp
07:17 Equilibrium Disturbance
07:45 How Concentration Affects Equilibrium
09:30 How Pressure/Volume Affects Equilibrium
10:54 How Temperature Affects Equilibrium
12:28 Microscopic vs Macroscopic Changes at Equilibrium
14:01 How Catalyst Affects Equilibrium
How Collision Theory Relates to Equilibrium // HSC Chemistry
Collision Theory
GCSE Chemistry - Factors Affecting the Rate of Reaction #47
Collision Theory & Reactions - Part 1 | Reactions | Chemistry | FuseSchool
Collision Theory & Reactions Part 1 | Reactions | Chemistry | FuseSchool
Collision theory | Kinetics | AP Chemistry | Khan Academy
Introduction to Collision Theory
4 Things about Collision Theory | Chemistry | #collisiontheory #shorts
Collision Theory & Maxwell Boltzmann #collision #chemistry #particles #energy #pressure #tempera...
Collision Theory on Bond Formation and Reaction Rates
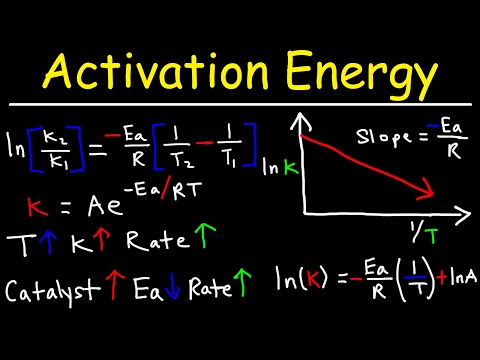
Collision Theory - Arrhenius Equation & Activation Energy - Chemical Kinetics
What is activation energy and collision theory?
What is collision theory? GCSE level explanation
R2.2.2 Collision theory
What is Collision Theory?
Collision Theory
Collision Theory: What basic behaviors are required of particles in order to react?
Collision Theory and Catalysts [IB Chemistry SL/HL]
Collision Theory and Reaction Rate // HSC Chemistry
Collision Theory grade 12: Part 1
Energy Diagrams, Catalysts, and Reaction Mechanisms
Collision Theory and Rate of Reaction
Chemistry SPM: Collision Theory In 5 Minutes
Collision Theory & Reactions - Part 2 | Reactions | Chemistry | FuseSchool
Комментарии
 0:15:42
0:15:42
 0:02:13
0:02:13
 0:05:15
0:05:15
 0:02:59
0:02:59
 0:02:29
0:02:29
 0:08:48
0:08:48
 0:03:35
0:03:35
 0:00:57
0:00:57
 0:00:59
0:00:59
 0:02:08
0:02:08
 0:31:50
0:31:50
 0:02:10
0:02:10
 0:01:53
0:01:53
 0:01:36
0:01:36
 0:00:08
0:00:08
 0:04:13
0:04:13
 0:02:10
0:02:10
 0:08:03
0:08:03
 0:14:57
0:14:57
 0:02:05
0:02:05
 0:05:23
0:05:23
 0:16:34
0:16:34
 0:05:26
0:05:26
 0:03:58
0:03:58