filmov
tv
Collision Theory

Показать описание
Learn about the three parts of collision theory and what it takes for a reaction to occur in this video!
Collision Theory
Collision theory | Kinetics | AP Chemistry | Khan Academy
Collision Theory & Reactions Part 1 | Reactions | Chemistry | FuseSchool
Collision Theory & Reactions - Part 1 | Reactions | Chemistry | FuseSchool
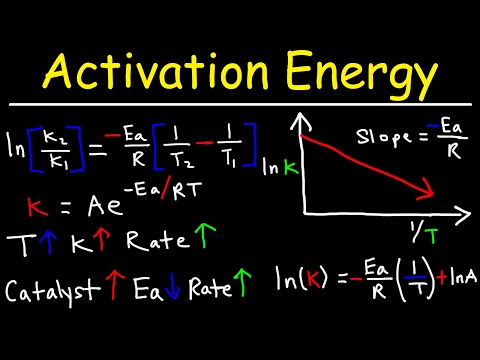
Collision Theory - Arrhenius Equation & Activation Energy - Chemical Kinetics
Elastic and Inelastic Collisions
Collision Theory on Bond Formation and Reaction Rates
GCSE Chemistry - Factors Affecting the Rate of Reaction #47
Did Earth Once Have Two Moons? Discover the Fascinating Lunar Theory! #facts #didyouknow #trivia
Collision Theory grade 12: Part 1
Energy Diagrams, Catalysts, and Reaction Mechanisms
How to speed up chemical reactions (and get a date) - Aaron Sams
Collision Theory
Collision Theory - Explained
Collision Theory: What basic behaviors are required of particles in order to react?
Collision Theory and Reaction Rate // HSC Chemistry
What is Collision Theory?
14.4 Collision Theory and the Arrhenius Equation | General Chemistry
Collision theory and the Maxwell–Boltzmann distribution | Kinetics | AP Chemistry | Khan Academy
3D Chemistry Lab - Collision Theory
Collision Theory and Rate of Reaction
DepEd Physical Science Module Week 5 Day 1, 2, 3 Collision Theory Reaction Rate and Catalysts
Collisions: Crash Course Physics #10
Collision Theory grade 12: Part 2
Комментарии
 0:02:13
0:02:13
 0:08:48
0:08:48
 0:02:29
0:02:29
 0:02:59
0:02:59
 0:31:50
0:31:50
 0:05:14
0:05:14
 0:02:08
0:02:08
 0:05:15
0:05:15
 0:00:40
0:00:40
 0:02:05
0:02:05
 0:05:23
0:05:23
 0:04:56
0:04:56
 0:03:50
0:03:50
 0:13:56
0:13:56
 0:02:10
0:02:10
 0:14:57
0:14:57
 0:00:08
0:00:08
 0:23:20
0:23:20
 0:07:28
0:07:28
 0:00:50
0:00:50
 0:16:34
0:16:34
 0:23:35
0:23:35
 0:09:21
0:09:21
 0:11:52
0:11:52