filmov
tv
What is Collision Theory?

Показать описание
Collision theory is a fundamental concept in chemistry and physics that explains the rates of chemical reactions and the factors that influence them. It provides insights into how reactant particles must collide in order to form products.
Key points about collision theory include:
1. Collision Requirement: For a chemical reaction to occur, reactant particles must collide with each other. However, not all collisions lead to a reaction. The collisions must occur with sufficient energy and proper orientation for the reaction to proceed.
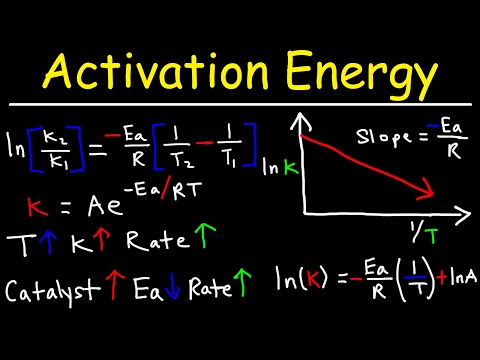
2. Activation Energy: In collision theory, activation energy refers to the minimum energy required for a successful reaction to take place. When colliding particles possess enough energy to overcome the activation energy barrier, bonds can break and new bonds can form, resulting in the formation of products.
3. Collision Frequency: The rate of collisions between reactant particles is crucial in determining the reaction rate. The higher the collision frequency, the more opportunities for successful collisions to occur. Factors such as concentration, pressure, and temperature affect collision frequency.
4. Collision Energy: The kinetic energy of colliding particles influences the likelihood of a successful collision. Higher kinetic energy leads to more energetic collisions, increasing the chances of overcoming the activation energy barrier and initiating a reaction.
5. Effective Collisions: Not all collisions result in a reaction. For a collision to be effective, it must occur with sufficient energy and the correct orientation. Only when the particles collide in the proper orientation can the existing bonds be broken and new bonds formed.
#sciencefacts #rigidbody #physics #scienceshorts #homeschoolcurriculum
Key points about collision theory include:
1. Collision Requirement: For a chemical reaction to occur, reactant particles must collide with each other. However, not all collisions lead to a reaction. The collisions must occur with sufficient energy and proper orientation for the reaction to proceed.
2. Activation Energy: In collision theory, activation energy refers to the minimum energy required for a successful reaction to take place. When colliding particles possess enough energy to overcome the activation energy barrier, bonds can break and new bonds can form, resulting in the formation of products.
3. Collision Frequency: The rate of collisions between reactant particles is crucial in determining the reaction rate. The higher the collision frequency, the more opportunities for successful collisions to occur. Factors such as concentration, pressure, and temperature affect collision frequency.
4. Collision Energy: The kinetic energy of colliding particles influences the likelihood of a successful collision. Higher kinetic energy leads to more energetic collisions, increasing the chances of overcoming the activation energy barrier and initiating a reaction.
5. Effective Collisions: Not all collisions result in a reaction. For a collision to be effective, it must occur with sufficient energy and the correct orientation. Only when the particles collide in the proper orientation can the existing bonds be broken and new bonds formed.
#sciencefacts #rigidbody #physics #scienceshorts #homeschoolcurriculum
Комментарии
 0:02:13
0:02:13
 0:02:29
0:02:29
 0:02:59
0:02:59
 0:08:48
0:08:48
 0:01:53
0:01:53
 0:05:15
0:05:15
 0:02:05
0:02:05
 0:02:36
0:02:36
 0:00:33
0:00:33
 0:02:28
0:02:28
 0:00:08
0:00:08
 0:31:50
0:31:50
 0:07:08
0:07:08
 0:02:08
0:02:08
 0:14:57
0:14:57
 0:08:03
0:08:03
 0:02:10
0:02:10
 0:13:56
0:13:56
 0:07:28
0:07:28
 0:02:10
0:02:10
 0:04:13
0:04:13
 0:03:26
0:03:26
 0:07:36
0:07:36
 0:03:50
0:03:50