filmov
tv
Effect of concentration on electrode potential values from www.ChemistryTuition.Net

Показать описание
Effect of concentration on electrode potential values from www.ChemistryTuition.Net
Effect of concentration on electrode potential
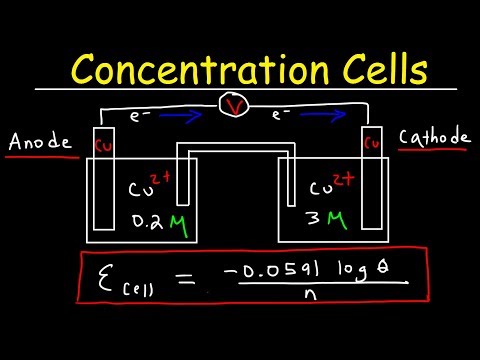
Concentration Cells & Cell Potential Calculations - Electrochemistry
Effect of Concentration change on EMF/ electrode potential || Class12 Electrochemistry #iitjee
04. Effect of Electrolyte concentration on electrode and cell potential
Variation of conductivity with dilution- Part 2 | Electrochemistry | Chemistry | Khan Academy
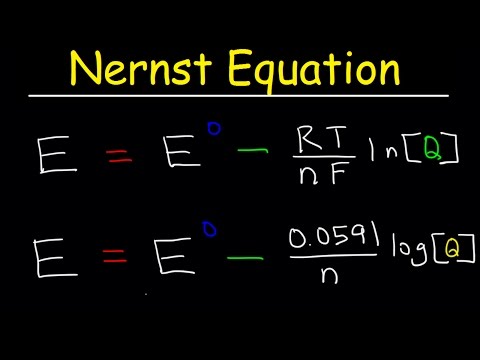
Nernst Equation Explained, Electrochemistry, Example Problems, pH, Chemistry, Galvanic Cell
Voltaic cell | How does it work?
19.5b Concentration Cells | General Chemistry
Determination of EMF of a Cell - MeitY OLabs
Overpotentials in Electrochemistry
What Is Electrolysis | Reactions | Chemistry | FuseSchool
Determination of Emf of a Cell - MeitY OLabs
Standard reduction potentials | Redox reactions and electrochemistry | Chemistry | Khan Academy
Factors Affecting the Preferential Discharge of Ions at Electrodes
[Ch 1.3c] Effects of Other Equilibria on Electrode Potentials
The Nernst Equation and Equilibrium Potentials in Physiology
Sulfuric Acid on Toilet Paper Spawns a Demon
Overpotential Explained
Cell Potential Problems - Electrochemistry
sulphuric acid #shorts
Electrochemistry | The Concentration Cell.
Factors Affecting Electrode Potential | Class 12 | Alakh Pandey Sir | @Alakh Sir Highlights
Electrolysis using salt experiment.
Комментарии
 0:10:07
0:10:07
 0:03:37
0:03:37
 0:14:22
0:14:22
 0:03:47
0:03:47
 0:25:57
0:25:57
 0:08:18
0:08:18
 0:30:53
0:30:53
 0:04:10
0:04:10
 0:08:44
0:08:44
 0:03:34
0:03:34
 0:06:29
0:06:29
 0:05:11
0:05:11
 0:02:44
0:02:44
 0:09:10
0:09:10
 0:09:01
0:09:01
![[Ch 1.3c] Effects](https://i.ytimg.com/vi/td5MmyjoxiM/hqdefault.jpg) 0:28:45
0:28:45
 0:10:31
0:10:31
 0:00:38
0:00:38
 0:06:36
0:06:36
 0:10:56
0:10:56
 0:00:17
0:00:17
 0:05:19
0:05:19
 0:01:46
0:01:46
 0:00:43
0:00:43