filmov
tv
Electrolysis using salt experiment.

Показать описание
Electrolysis using salt experiment.
Electrolysis of Salt Water - Aqueous Compounds | GCSE Chemistry
Electrolysis of Salt Solution at Home in Under 1 minute to produce Hydrogen and Chlorine
Electrolysis Science Experiment of Water and Salt // Easy Science Fair Project for Students
How To Produce Oxygen Gas At Home/OXYGEN AND HYDROGEN From Water | Electrolysis Of Water At Home
Electrolysis with salt water experiment 😱#viral #shorts #tranding
WATER ELECTROLYSIS DEMONSTRATION WITH EXPLANATION | CHEMISTRY | GRADE 8-12
Electrolysis Of Water | How To Produce Hydrogen From Water | Water Electrolysis #shorts
Simple demonstration of electrolysis of water (SEE UPDATED VERSION LINK IN DESCRIPTION)
Electrical Conductivity with salt water & sugar water
Electrolysis: Producing hydrogen from water
Electrolysis Science Experiment with salt water
Standard Zinc-Copper Voltaic Cell with Salt Bridge
Electrolysis of salt water using copper & zinc
Electrolysis Of Sodium Chloride
Simple and easy science experiments.Electrolysis of water with NaCl.#shorts #diy #experiment #dwe .
Electrolysis of water science project | Electrolys Using Salt | #experiment
water vs salt🤔 /Electrolysis using salt water experiment 😱#shorts #experiment
Electrolysis of salt water; simple home experiment
Water vs Battery || H2 and O2 out from water || Water Electrolysis Gas #science #experiment
The Sci Guys: Science at Home - SE1 - EP1: Electrolysis of Water
Electrolysis | Pencil + Salt Water + 9v Battery Experiment #shorts #experiment
How To Produce Hydrogen And Oxygen From Water/ Electrolysis Of Water, #shorts
Electrolysis for beginners! Choose Hydrogen not Chlorine.
Комментарии
 0:00:43
0:00:43
 0:03:14
0:03:14
 0:01:00
0:01:00
 0:02:46
0:02:46
 0:04:36
0:04:36
 0:00:33
0:00:33
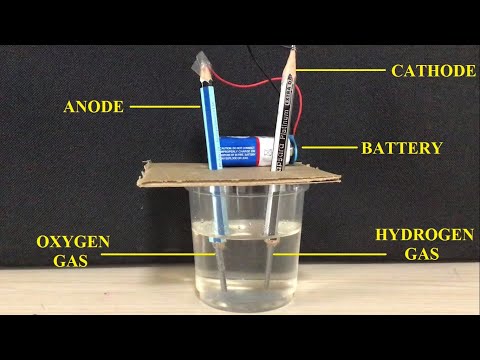 0:10:31
0:10:31
 0:00:25
0:00:25
 0:02:49
0:02:49
 0:01:52
0:01:52
 0:00:54
0:00:54
 0:01:00
0:01:00
 0:04:52
0:04:52
 0:04:27
0:04:27
 0:04:10
0:04:10
 0:00:15
0:00:15
 0:02:44
0:02:44
 0:00:46
0:00:46
 0:03:16
0:03:16
 0:00:54
0:00:54
 0:07:26
0:07:26
 0:00:20
0:00:20
 0:00:56
0:00:56
 0:11:30
0:11:30