filmov
tv
Collision Theory and Activation Energy (A-Level IB Chemistry)

Показать описание
Outlining collision theory and activation energy, showing how they link to rates of reactions. The effect of temperature change, pressure change and concentration change is shown with examples, including hydrogen and chlorine forming hydrogen chloride.
Recap: 00:38
Collision Theory: 02:25
Activation Energy: 03:01
Successful and Unsuccessful Collisions: 04:03
Example - Hydrogen and Chlorine forming Hydrogen Chloride: 04:33
Effect of Temperature on Rates of Reaction: 06:05
Effect of Concentration on Rates of Reaction: 07:09
Effect of Pressure on Rates of Reaction: 09:01
Catalysts: 09:32
Summary: 11:57
Relevant Videos:
Thank you for watching - if you found the video useful, please like and subscribe!
Recap: 00:38
Collision Theory: 02:25
Activation Energy: 03:01
Successful and Unsuccessful Collisions: 04:03
Example - Hydrogen and Chlorine forming Hydrogen Chloride: 04:33
Effect of Temperature on Rates of Reaction: 06:05
Effect of Concentration on Rates of Reaction: 07:09
Effect of Pressure on Rates of Reaction: 09:01
Catalysts: 09:32
Summary: 11:57
Relevant Videos:
Thank you for watching - if you found the video useful, please like and subscribe!
What is activation energy and collision theory?
Collision Theory
Collision Theory - Arrhenius Equation & Activation Energy - Chemical Kinetics
Collision theory | Kinetics | AP Chemistry | Khan Academy
GCSE Chemistry - Factors Affecting the Rate of Reaction #47
Collision Theory and Activation Energy (A-Level IB Chemistry)
Activation Energy
Collision theory and activation energy
Formula Firestorm: Ignite Your GATE 2025 Preparations Part 2 | Chemical Reaction Engineering
Energy Diagrams, Catalysts, and Reaction Mechanisms
Collision Theory and Activation Energy
Effective collision and activation energy
What is collision theory? GCSE level explanation
14.4 Collision Theory and the Arrhenius Equation | General Chemistry
Chemistry Chapter 3 (3.2) collision theory and activation energy အစအဆုံး by Sayar Kaung...
HSC Collision Theory and activation energy
Collision theory vs activation energy | U2 | ATAR Chemistry QCE
Kinetics: Chemistry's Demolition Derby - Crash Course Chemistry #32
Collision theory and the Maxwell–Boltzmann distribution | Kinetics | AP Chemistry | Khan Academy
What is Collision Theory?
Collision Theory and Activation Energy Notes.mp4
Chemistry Class 11 | Chapter 9 | Topic 7 | Collision Theory, Transition State and Activation Energy
Collision Theory: Why Do Chemical Reactions Happen?
14.4 Collision Theory and the Arrhenius Equation
Комментарии
 0:02:10
0:02:10
 0:02:13
0:02:13
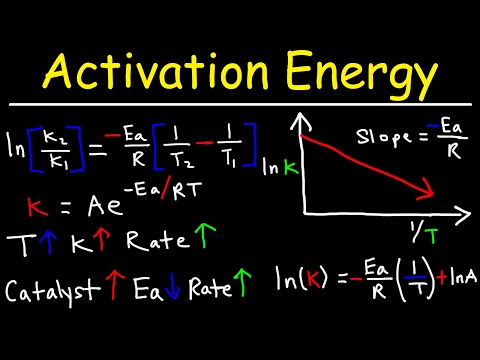 0:31:50
0:31:50
 0:08:48
0:08:48
 0:05:15
0:05:15
 0:14:12
0:14:12
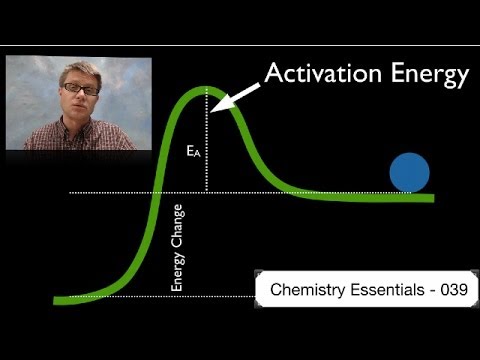 0:04:52
0:04:52
 0:03:13
0:03:13
 1:03:13
1:03:13
 0:05:23
0:05:23
 0:01:06
0:01:06
 0:01:42
0:01:42
 0:01:53
0:01:53
 0:23:20
0:23:20
 0:38:09
0:38:09
 0:08:07
0:08:07
 0:02:14
0:02:14
 0:09:57
0:09:57
 0:07:28
0:07:28
 0:00:08
0:00:08
 0:14:37
0:14:37
 0:06:30
0:06:30
 0:00:10
0:00:10
 0:12:57
0:12:57