filmov
tv
7.7 How to Distinguish Between Substitution and Elimination Reactions (SN2 SN1 E2 E1) | OChem

Показать описание
Chad begins this lesson comparing SN2, SN1, E2, and E1 reactions. Based upon the differences between substitution and elimination reactions, he then develops the method for how to predict the products of substitution and elimination reactions and how to distinguish between SN2, SN1, E2, and E1 reactions. SN1 and SN2 reactions were covered at length earlier in the chapter with a lesson showing how the nucleophile, substrate, and solvent can be used to predict the major substitution product (SN1 vs SN2). E1 and E2 reactions were also covered at length with a lesson showing how the base, substrate, and solvent can be used to predict the major elimination product (E1 vs E2). Now, Chad compares and contrasts SN2, SN1, E2, and E1 reactions simultaneously in terms of the nucleophile/base, substrate, and solvent and how the differences can be used to predict which of these reactions are likely to occur so that the major and minor products can be determined. He provides an SN1, SN2, E1, E2 chart that outlines what substitution and/or elimination reaction mechanism is likely based upon the combination of the particular substrate and nucleophile/base being used. Chad works five substitution and elimination practice problems to demonstrate how the chart is used to determine the major products.
00:00 Lesson Introduction
00:36 Comparing and Contrasting SN1, SN2, E1, E2
04:22 How to Predict the Products Example #1
06:13 How to Predict the Products Example #2
08:06 How to Predict the Products Example #3
09:17 How to Predict the Products Example #4
11:10 How to Predict the Products Example #5
00:00 Lesson Introduction
00:36 Comparing and Contrasting SN1, SN2, E1, E2
04:22 How to Predict the Products Example #1
06:13 How to Predict the Products Example #2
08:06 How to Predict the Products Example #3
09:17 How to Predict the Products Example #4
11:10 How to Predict the Products Example #5
Комментарии
 0:14:26
0:14:26
 0:01:57
0:01:57
 0:01:00
0:01:00
 0:05:32
0:05:32
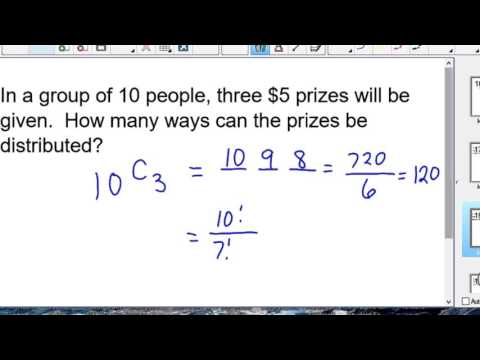 0:08:51
0:08:51
 0:03:27
0:03:27
 0:09:03
0:09:03
 0:03:46
0:03:46
 0:07:40
0:07:40
 0:13:40
0:13:40
 0:03:41
0:03:41
 0:03:49
0:03:49
 0:05:40
0:05:40
 0:06:28
0:06:28
 0:02:28
0:02:28
 0:05:53
0:05:53
 0:02:21
0:02:21
 0:03:39
0:03:39
 0:00:46
0:00:46
 0:03:26
0:03:26
 0:04:13
0:04:13
 0:02:12
0:02:12
 0:04:53
0:04:53
 0:03:52
0:03:52