filmov
tv
Tracing the Evolution of Atomic Models Through History: #quantum #sciencex #schrödinger #research

Показать описание
1. Dalton’s Atomic Model (1803): John Dalton proposed that matter is made up of small, indivisible particles called atoms. According to this model, atoms of the same element are identical in mass and properties, while atoms of different elements differ in these characteristics. Dalton visualized atoms as solid, indivisible spheres, without any information about their internal structure.
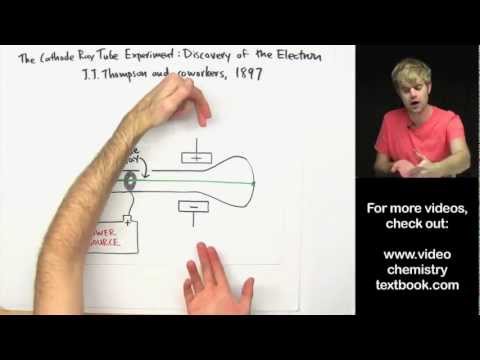
2. Thomson’s Plum Pudding Model (1904): J.J. Thomson, following his discovery of the electron, suggested that an atom consists of a positively charged sphere with negatively charged electrons embedded within it. This arrangement was likened to a "plum pudding," where the electrons represented the plums, and the positively charged sphere was the pudding.
3. Rutherford’s Nuclear Model (1911): Ernest Rutherford’s gold foil experiment led to the discovery that an atom has a small, dense, positively charged nucleus at its center, with electrons revolving around it in orbits. This model replaced Thomson’s plum pudding model and introduced the concept of a nuclear atom with a central nucleus.
4. Bohr’s Model (1913): Niels Bohr proposed that electrons move around the nucleus in fixed, circular orbits, called energy levels, and they do not emit energy while remaining in these orbits. This model introduced the concept of quantized energy levels for electrons and marked a significant advancement in quantum theory.
5. Schrödinger’s Quantum Mechanical Model (1926): Erwin Schrödinger used #quantum mechanics to develop a model where electrons do not follow defined paths but instead exist in cloud-like regions called orbitals. This model also incorporated Heisenberg’s uncertainty principle, which states that the exact position and momentum of an electron cannot be simultaneously determined. #Schrödinger’s model forms the foundation of modern atomic theory.
#ScienceX #scienceolympiads #sciencexnews #sciencexeducation #sciencexpublications #science #scienceforthecurious #sciencexchampionship#sciencexchallange #science #sciencex #Research
2. Thomson’s Plum Pudding Model (1904): J.J. Thomson, following his discovery of the electron, suggested that an atom consists of a positively charged sphere with negatively charged electrons embedded within it. This arrangement was likened to a "plum pudding," where the electrons represented the plums, and the positively charged sphere was the pudding.
3. Rutherford’s Nuclear Model (1911): Ernest Rutherford’s gold foil experiment led to the discovery that an atom has a small, dense, positively charged nucleus at its center, with electrons revolving around it in orbits. This model replaced Thomson’s plum pudding model and introduced the concept of a nuclear atom with a central nucleus.
4. Bohr’s Model (1913): Niels Bohr proposed that electrons move around the nucleus in fixed, circular orbits, called energy levels, and they do not emit energy while remaining in these orbits. This model introduced the concept of quantized energy levels for electrons and marked a significant advancement in quantum theory.
5. Schrödinger’s Quantum Mechanical Model (1926): Erwin Schrödinger used #quantum mechanics to develop a model where electrons do not follow defined paths but instead exist in cloud-like regions called orbitals. This model also incorporated Heisenberg’s uncertainty principle, which states that the exact position and momentum of an electron cannot be simultaneously determined. #Schrödinger’s model forms the foundation of modern atomic theory.
#ScienceX #scienceolympiads #sciencexnews #sciencexeducation #sciencexpublications #science #scienceforthecurious #sciencexchampionship#sciencexchallange #science #sciencex #Research
Комментарии
 0:00:54
0:00:54
 0:04:26
0:04:26
 0:05:23
0:05:23
 0:07:04
0:07:04
 0:47:47
0:47:47
 0:09:35
0:09:35
 0:05:17
0:05:17
 0:00:52
0:00:52
 0:11:08
0:11:08
 0:03:05
0:03:05
 0:19:07
0:19:07
 0:03:28
0:03:28
 0:00:42
0:00:42
 0:45:42
0:45:42
 0:15:24
0:15:24
 0:01:00
0:01:00
 0:16:27
0:16:27
 0:03:42
0:03:42
 0:00:50
0:00:50
 0:03:22
0:03:22
 0:04:07
0:04:07
 0:03:57
0:03:57
 0:04:31
0:04:31
 0:03:19
0:03:19