filmov
tv
Using Thermodynamic Relationships
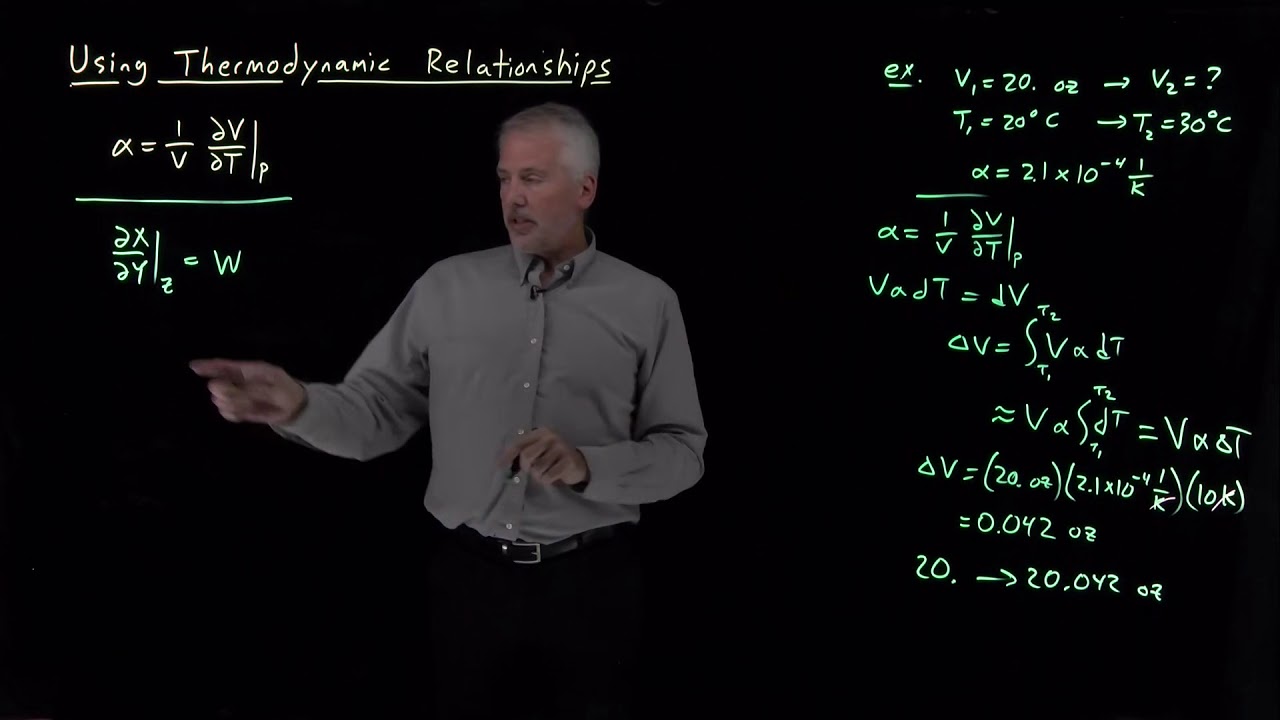
Показать описание
The key to solving many thermodynamics problems is being able to identify which thermodynamic derivative is needed.
Using Thermodynamic Relationships
Thermodynamic Relationships
Trick to find maxwell relation by thermodynamic square ..MWE #shorts #youtubeshorts #youtube
The First Law of Thermodynamics: Internal Energy, Heat, and Work
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
Thermodynamic Relations, What are?
Mnemonic Device For Thermodynamic Potentials and Maxwell's Relations
Mastering Ensembles: Microcanonical, Canonical & Grand Canonical for IIT JAM, JEST, GATE & C...
Trick to find Thermodynamic Relations #shorts #chemistry #education #neet #reels
Simplest Trick (Mnemonic) for all THERMODYNAMIC RELATIONS (Maxwell Relation)
Maxwell thermodynamic relations
Thermodynamic Square to find the Fundamental Property Relations & Maxwell Relations
Thermodynamic Properties: They're All Related
Thermodynamic relation Trick || Thermodynamic potentials and variables ||IIT JAM Problem & solut...
Lesson 3A Thermodynamic Relationships with Entropy
Learn Thermodynamic Relations through mnemonic. Nerdy lecture series 1
MAXWELL'S THERMODYNAMIC RELATION .#Short trick must watch .
Second Law of Thermodynamics - Heat Energy, Entropy & Spontaneous Processes
Tds Equation । Using Maxwell Thermodynamic Relation ।Bsc Physics । Hindi-English
Maxwell's relations - Thermodynamic square shortcut method - easy trick - derivation of equatio...
Hess's Law Problems & Enthalpy Change - Chemistry
YOU WILL NOT FORGET MAXWELL’S THERMODYNAMIC RELATIONS WITH THIS TRICK | PHYSICS HUB
Easy To Deduce Maxwell Four Thermodynamic Relations
Комментарии
 0:09:05
0:09:05
 0:03:48
0:03:48
 0:00:41
0:00:41
 0:05:44
0:05:44
 0:08:12
0:08:12
 0:11:27
0:11:27
 0:02:28
0:02:28
 0:11:20
0:11:20
 0:54:21
0:54:21
 0:01:00
0:01:00
 0:24:40
0:24:40
 0:00:16
0:00:16
 0:08:39
0:08:39
 0:14:26
0:14:26
 0:07:56
0:07:56
 0:04:49
0:04:49
 0:05:46
0:05:46
 0:00:07
0:00:07
 0:04:11
0:04:11
 0:11:16
0:11:16
 0:13:04
0:13:04
 0:14:03
0:14:03
 0:07:12
0:07:12
 0:00:38
0:00:38