filmov
tv
Measuring the specific latent heat of vaporization of water

Показать описание
In this video, we shall talk about an experiment to measure the specific latent heat of vaporization of water. We shall introduce the experimental setup and mention how the limitations of the experiment will affect the final result.
Measuring the specific latent heat of fusion of Ice
N5 Physics. Specific Latent Heat of Fusion of ice
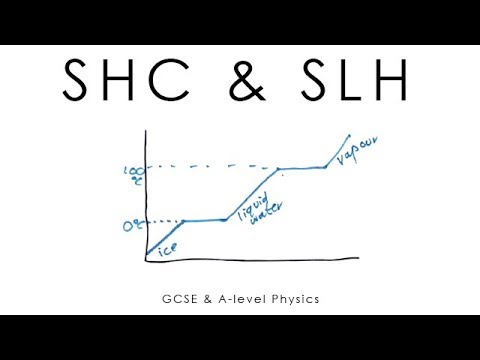
GCSE Physics - Specific Latent Heat #29
Specific Latent Heat | Matter | Physics | FuseSchool
Measuring the Specific Latent Heat of Fusion of water - method 1
Finding Specific Latent Heat of Water - GCSE & A-Level Physics Practical
Thermodynamics: Calculating Latent and Specific Heat, Example Problem
A Level Physics: Specific Latent Heat
O level physics (6091) 2020 paper 1 | TYS Past Year Paper Full Solution!
Heat Capacity, Specific Heat, and Calorimetry
GCSE Physics: Measuring Specific Latent Heat of Fusion of Ice
Specific Latent Heat of Water - A Level Physics Practical
Specific Heat Capacity - GCSE Science Required Practical
Measuring the specific latent heat of vaporization of water
Latent heat of fusion of ice
Specific Heat Capacity and Latent Heat Calculations
GCSE Physics - Internal Energy and Specific Heat Capacity #28
P1.11 Measuring Specific Latent Heat
GCE A Level Physics | Specific Latent Heat and Latent Heat (Thermal Physics Chapter)
Specific Heat Capacity + Latent Heat - GCSE & A-level Physics (full version)
Finding the specific latent heat using electrical methods - Lab Experiment
Measuring the Specific Latent Heat of Fusion of water - method 2
Specific Heat Capacity of Water - Physics Experiment
GCSE Physics with Lewis (Specific Latent Heat) -6th May 2020
Комментарии
 0:04:44
0:04:44
 0:06:32
0:06:32
 0:06:26
0:06:26
 0:03:55
0:03:55
 0:07:23
0:07:23
 0:02:17
0:02:17
 0:06:46
0:06:46
 0:03:24
0:03:24
 1:23:35
1:23:35
 0:04:14
0:04:14
 0:08:40
0:08:40
 0:07:48
0:07:48
 0:07:04
0:07:04
 0:08:37
0:08:37
 0:04:51
0:04:51
 0:03:46
0:03:46
 0:04:36
0:04:36
 0:06:10
0:06:10
 0:03:17
0:03:17
 0:13:53
0:13:53
 0:05:09
0:05:09
 0:08:03
0:08:03
 0:03:11
0:03:11
 0:25:17
0:25:17