filmov
tv
Thermodynamics: Calculating Latent and Specific Heat, Example Problem
Показать описание
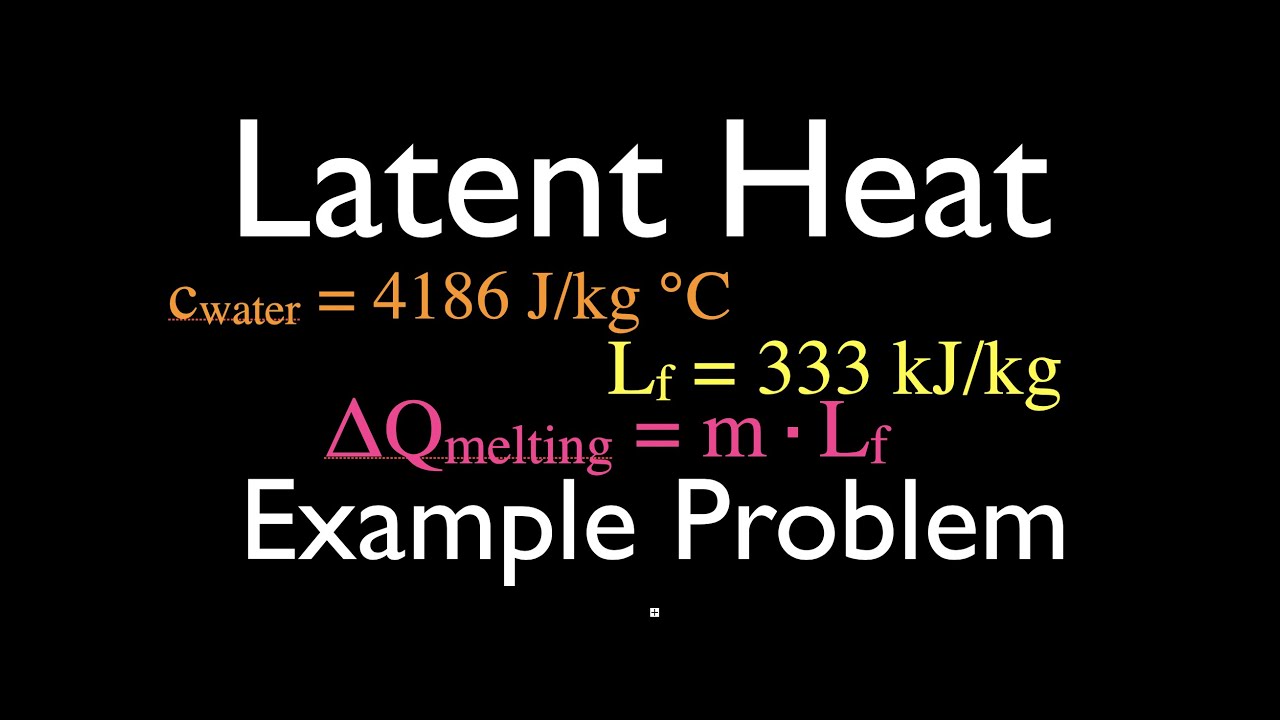
This video show how to calculate the heat needed take solid ice, melt it and then raise the temperature of the liquid water.
Latent heat is the heat added to or removed from a material to change the phase of the material without changing its temperature. Examples are latent heat of fusion which is the heat needed to melt or freeze a substance and latent heat of vaporization which is the heat involved in vaporizing or condensing a material.
Specific heat it is the amount of energy that must be added to or removed from one unit of mass of a substance (usually a kg or g) in order to cause an increase of one degree celsius (or Kelvin).
Social Media for Step by Step Science:
Latent heat is the heat added to or removed from a material to change the phase of the material without changing its temperature. Examples are latent heat of fusion which is the heat needed to melt or freeze a substance and latent heat of vaporization which is the heat involved in vaporizing or condensing a material.
Specific heat it is the amount of energy that must be added to or removed from one unit of mass of a substance (usually a kg or g) in order to cause an increase of one degree celsius (or Kelvin).
Social Media for Step by Step Science:
Thermodynamics: Calculating Latent and Specific Heat, Example Problem
GCSE Physics - Specific Latent Heat #29
Specific Heat Capacity and Latent Heat Calculations
Latent Heat and Sensible Heat Explained | Humidity | Animation | #hvac #hvacsystem #hvacmaintenance
Heat Capacity, Specific Heat, and Calorimetry
Specific heat and latent leat of fusion and vaporization | Chemistry | Khan Academy
Specific Heat And Latent Heat | Change Of State And Calorimetry | Question 3 | Thermodynamics
Specific and Latent Heat
Latent Heat 😦
Latent Heat Exercise #1 of 3 // Thermodynamics - Class 103
11. Sensible and Latent Heat Equation.
Example Latent Heat Calculation AICHS
Latent Heat Equations
Solving Heat Capacity and Specific Heat Capacity problems - Pure Physics
Specific Heat and Latent Heat
Latent heat calculation Part 1 | SPM
Phase Changes, Heats of Fusion and Vaporization, and Phase Diagrams
Latent Heat Example # 2
Specific Heat and Latent Heat of fusion and vaporization
Finding the specific latent heat using electrical methods - Lab Experiment
Specific Heat Capacity + Latent Heat - GCSE & A-level Physics (full version)
Latent Heat Exercise #2 of 3 // Thermodynamics - Class 103
Latent Heat and Thermodynamics: Step-by-Step Problem Solving
Latent Heat Example # 1
Комментарии
 0:06:46
0:06:46
 0:06:26
0:06:26
 0:03:46
0:03:46
 0:08:03
0:08:03
 0:04:14
0:04:14
 0:14:57
0:14:57
 0:17:50
0:17:50
 0:12:55
0:12:55
 0:00:35
0:00:35
 0:06:07
0:06:07
 0:15:07
0:15:07
 0:04:44
0:04:44
 0:07:48
0:07:48
 0:03:53
0:03:53
 0:04:30
0:04:30
 0:10:24
0:10:24
 0:04:51
0:04:51
 0:06:42
0:06:42
 0:14:57
0:14:57
 0:05:09
0:05:09
 0:13:53
0:13:53
 0:04:32
0:04:32
 0:21:19
0:21:19
 0:03:16
0:03:16