filmov
tv
Quick Revision - Enthalpy change of neutralisation

Показать описание
Description of the essentials followed by the practical procedure and a typical calculation.
Quick Revision - AS Enthalpy Changes
Hess's Law Problems & Enthalpy Change - Chemistry
Enthalpy Changes [IB Chemistry SL/HL]
Quick Revision - Enthalpy changes from equations
OCR A 3.2.1 Enthalpy changes REVISION
Quick Revision - Enthalpy changes from bond enthalpies
The EASIEST Method For Solving Hess Cycles
Every Enthalpy Change Animated & Explained IN UNDER 20 MINUTES
Quick Revision - Vector approach to Hess' Law using enthalpy changes of formation
Quick Revision - Enthalpies of solution
Enthalpy Changes A* Revision OCR A-Level Chemistry
Enthalpy Change Definitions
Thermochemistry: Heat and Enthalpy
Enthalpy: Crash Course Chemistry #18
Calculating enthalpy change
Quick Revision - Enthalpy change of neutralisation
Did you know how to remember reactivity series?
Enthalpy Diagrams
Quick Revision - Hess' Law cycles (formation)
AQA 1.4 Energetics REVISION
Enthalpy | Thermodynamics
periodic table chemistry wali bhabhi ke yad Hain #chemistry
Bond Energy Calculations & Enthalpy Change Problems, Basic Introduction, Chemistry
Quick review - Hess's Law and bond enthalpies
Комментарии
 0:04:01
0:04:01
 0:14:03
0:14:03
 0:11:56
0:11:56
 0:06:29
0:06:29
 0:23:26
0:23:26
 0:05:15
0:05:15
 0:13:46
0:13:46
 0:19:55
0:19:55
 0:03:43
0:03:43
 0:06:28
0:06:28
 0:09:19
0:09:19
 0:06:32
0:06:32
 0:04:17
0:04:17
 0:11:24
0:11:24
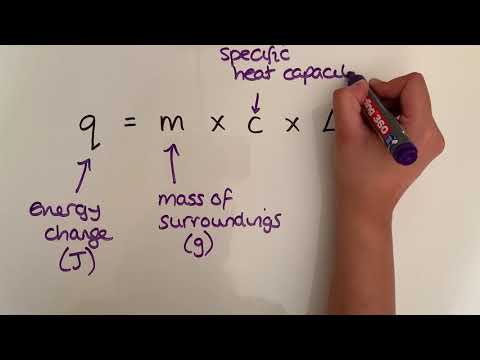 0:07:22
0:07:22
 0:00:30
0:00:30
 0:10:33
0:10:33
 0:04:01
0:04:01
 0:21:33
0:21:33
 0:10:55
0:10:55
 0:00:25
0:00:25
 0:11:39
0:11:39
 0:16:23
0:16:23