filmov
tv
Molecular Structure of CO2 (Carbon Dioxide)

Показать описание
There are a few ways we can think about the molecular structure of CO2. Looking at the chemical formula for CO2 we could draw an initial formula like this: O C O. We put C in the center of the structure since it is the least electronegative element.
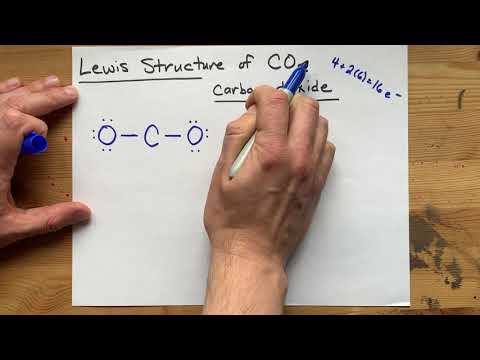
We could also draw a Lewis Structure for CO2 to describe where the valence electrons are around the structure of water. It gives us a lot more information and allow us to look at the molecular geometry for Carbon dioxide (CO2).
We see the chemical bonds between atoms where electron pairs are shared in the structure, and we see the pairs of unbonded electrons as well. But we still don’t know the three dimensional structure for CO2. For that we’ll use VSEPR theory.
Atoms are surrounded by valence electrons. These electrons clouds will push away from each other. Same goes for the unbonded electron pairs. Base on this information we can see that the molecule geometry for CO2 is linear and the bond angle is 180 degrees.
---Resources---
We could also draw a Lewis Structure for CO2 to describe where the valence electrons are around the structure of water. It gives us a lot more information and allow us to look at the molecular geometry for Carbon dioxide (CO2).
We see the chemical bonds between atoms where electron pairs are shared in the structure, and we see the pairs of unbonded electrons as well. But we still don’t know the three dimensional structure for CO2. For that we’ll use VSEPR theory.
Atoms are surrounded by valence electrons. These electrons clouds will push away from each other. Same goes for the unbonded electron pairs. Base on this information we can see that the molecule geometry for CO2 is linear and the bond angle is 180 degrees.
---Resources---
 0:02:28
0:02:28
 0:02:51
0:02:51
 0:00:56
0:00:56
 0:06:45
0:06:45
 0:04:08
0:04:08
 0:02:05
0:02:05
 0:06:10
0:06:10
 0:06:54
0:06:54
 0:00:17
0:00:17
 0:02:19
0:02:19
 0:01:45
0:01:45
 0:06:28
0:06:28
 0:04:55
0:04:55
 0:02:40
0:02:40
 0:01:27
0:01:27
 0:02:57
0:02:57
 0:03:32
0:03:32
 0:00:15
0:00:15
 0:01:00
0:01:00
 0:00:21
0:00:21
 0:00:55
0:00:55
 0:03:35
0:03:35
 0:23:07
0:23:07
 0:04:17
0:04:17