filmov
tv
Lewis Structure of CO2 (carbon dioxide)

Показать описание
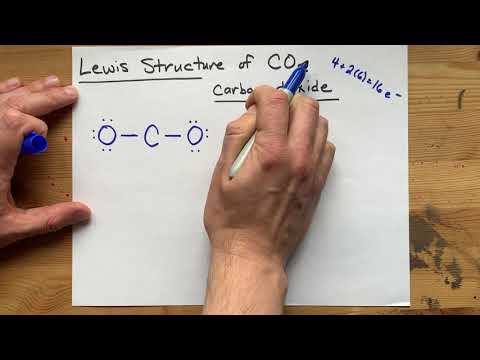
Carbon needs two double bonds, one to each of the two oxygens, to complete its octet. The atoms *share* electrons with each other because they are both non-metals (covalent bonding). The results is a LINEAR molecules, VSEPR notation AX2, bond angle 180 degrees.
Lewis Structure of CO2 (carbon dioxide)
CO2 Lewis Structure - Carbon Dioxide
Lewis structure of carbon dioxide #chemistry#shorts#lewisdotstructure#chemicalbonding
Lewis Dot Structure for Carbon dioxide
Molecular Structure of CO2 (Carbon Dioxide)
Carbon Dioxide Lewis Structure: How to Draw the Lewis Structure for Carbon Dioxide
Lewis structure of CO2 molecule step by step||Lewis structure of carbon dioxide
How do you draw Lewis Structure of CO2 (Carbon dioxide)?
Lewis structure for CO2 (Carbon dioxide)
Lewis Structure of CO2 (Carbon dioxide)||co2 lewis structure||How to Do CO2 Lewis Structure
How to Draw Lewis Structure for Carbon Dioxide (CO2) I Easy & Quick
Lewis structure of CO2 || Easy steps to draw Lewis diagrams Of CO2
Lewis Dot Structure for CO2 (Carbon Dioxide) #CO2LewisStructure ||Carbon Dioxide Lewis Structure
Lewis Structure Of CO2 | Lewis Structures | Organic Chemistry
The Lewis Dot Structure for Carbon Dioxide
How do you write the Lewis dot structure for CO2?
How to draw a lewis structure for CO2
CO2 Lewis Structure -How to Draw the Dot Structure for Carbon Dioxide(CO2)-Lewis Structure of CO2
How To Draw Lewis Structures
Orbital Overlap Diagram of CO2 (carbon dioxide)
Electron dot structure of CO2 l carbon dioxide electron dot structure
Lewis Structure of CO2
CO2 Lewis Structure - How to Draw or Write the Lewis Dot Structure for Carbon Dioxide,CO2
[FSH ED] Lewis Structure of Carbon Dioxide (CO2)
Комментарии
 0:02:51
0:02:51
 0:04:08
0:04:08
 0:00:56
0:00:56
 0:02:40
0:02:40
 0:02:28
0:02:28
 0:01:23
0:01:23
 0:04:55
0:04:55
 0:06:28
0:06:28
 0:03:01
0:03:01
 0:04:17
0:04:17
 0:03:28
0:03:28
 0:02:57
0:02:57
 0:07:12
0:07:12
 0:02:27
0:02:27
 0:05:22
0:05:22
 0:04:22
0:04:22
 0:03:40
0:03:40
 0:00:16
0:00:16
 0:11:50
0:11:50
 0:06:54
0:06:54
 0:00:55
0:00:55
 0:02:51
0:02:51
 0:04:39
0:04:39
![[FSH ED] Lewis](https://i.ytimg.com/vi/2jb6eRXlBXY/hqdefault.jpg) 0:06:54
0:06:54