filmov
tv
12 From Molecules to Solids

Показать описание
Molecular orbitals, Band theory, Band structure
12 From Molecules to Solids
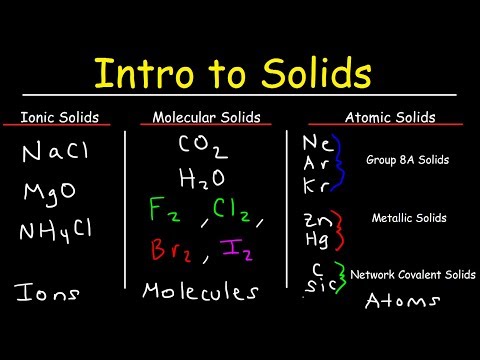
Ionic Solids, Molecular Solids, Metallic Solids, Network Covalent Solids, & Atomic Solids
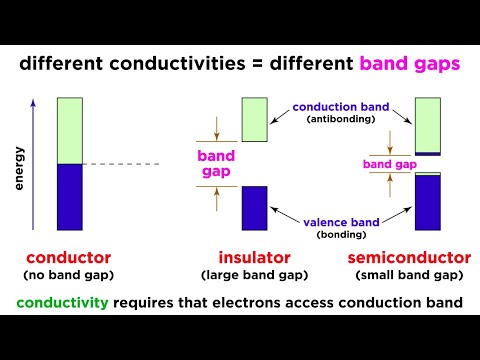
Conductivity and Semiconductors
11. Shapes of Molecules and VSEPR (Intro to Solid-State Chemistry)
VSEPR Theory and Molecular Geometry
Doing Solids: Crash Course Chemistry #33
Phys103 - Molecules and Solids (Molecular Spectra: Combining rotational and vibrational modes)
model of molecules of solid, liquid and gas
𝐒𝐎𝐋𝐈𝐃 𝐒𝐓𝐀𝐓𝐄 𝐏𝐇𝐘𝐒𝐈𝐂𝐒 (𝐅𝐫𝐞𝐞 𝐄𝐥𝐞𝐜𝐭𝐫𝐨𝐧 𝐓𝐡𝐞𝐨𝐫𝐲) 𝐋-𝟏𝟐 | 𝐓𝐀𝐑𝐆𝐄𝐓 𝐂𝐒𝐈𝐑 𝐍𝐄𝐓 𝐉𝐔𝐍𝐄 𝟐𝟎𝟐𝟓 | 𝐈𝐅𝐀𝐒...
Crystalline and Amorphous Solids
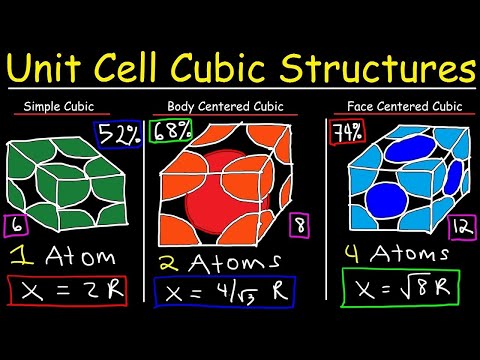
Unit Cell Chemistry Simple Cubic, Body Centered Cubic, Face Centered Cubic Crystal Lattice Structu
What Is Matter? - The Dr. Binocs Show | Best Learning Videos For Kids | Peekaboo Kidz
Solids, liquids and gases of water molecules
Polar and Non-polar Molecules. B.Sc. Second Year. Class 12. #shorts
Amazing molecules with incredible symmetry -S4N4
GCSE Physics - Particle Theory & States of Matter
Solid Molecules
States of Matter : Solid Liquid Gas
Vibratory Motion of molecules
Intermolecular Forces and Boiling Points
Arrangement of molecules in solid, liquid and gases| Science Activity
AS Chemistry 🧪 Tetrahedral molecules 💠
Trick to learn shapes of molecules | Geometry of molecules | VSEPR Theory
Kinetic Molecular Theory and its Postulates
Комментарии
 0:56:28
0:56:28
 0:20:19
0:20:19
 0:06:32
0:06:32
 0:48:42
0:48:42
 0:06:31
0:06:31
 0:09:18
0:09:18
 0:01:50
0:01:50
 0:00:15
0:00:15
 1:38:06
1:38:06
 0:07:47
0:07:47
 0:17:22
0:17:22
 0:07:19
0:07:19
 0:01:50
0:01:50
 0:00:16
0:00:16
 0:00:16
0:00:16
 0:04:34
0:04:34
 0:00:04
0:00:04
 0:14:28
0:14:28
 0:00:31
0:00:31
 0:10:54
0:10:54
 0:00:37
0:00:37
 0:00:47
0:00:47
 0:06:35
0:06:35
 0:07:00
0:07:00