filmov
tv
Sn2 Reactions

Показать описание
Sn2 Reactions
SN2 Reaction Mechanisms
Nucleophiles, Electrophiles, Leaving Groups, and the SN2 Reaction
SN2 Reactions | University Of Surrey
7.1 SN2 Reaction | Organic Chemistry
SN2 Reactions
Nucleophilic Substitution Reactions | SN1 Reaction and SN2 Reaction
Substitution Reactions - SN1 and SN2 Mechanisms: Crash Course Organic Chemistry #21
Sn2 reactions | Substitution and elimination reactions | Organic chemistry | Khan Academy
6,MHT CET PYQ,HALOGEN DERIVATIVES,SN1 AND SN2 REACTION,CHEMICAL REACTION OF HALOALKANE
Sn2 Reactions
Choosing Between SN1/SN2/E1/E2 Mechanisms
SN1 SN2 E1 E2 Reaction Mechanism Overview
SN2 SN1 E1 E2 Reaction Mechanisms Made Easy!
SN2 Reaction Rate and Mechanism (vid 1 of 3) Bimolecular Substitution by Leah4sci
7 SN1 vs SN2 Reactions
SN2 Practice Questions
How Do SN2 Reactions Work? (Animation) Organic Chemistry Substitution Mechanism
Ch#17 |Lec#3 | SN1 and SN2 Reactions & mechanism, Nucleophilic substitution Reactions
SN1 vs SN2 Reactions!
SN2 Reaction Rate!
Question 1: Is this an SN1 or SN2 reaction? #organicchemistry
Choosing Between SN2, SN1, E2 and E1 Reactions
SN1 and SN2 Mechanisms (A-level Chemistry)
difference between SN1 and SN2 reaction #chemistry #organicchemistry #bscchemistry #ytshorts #yt
Комментарии
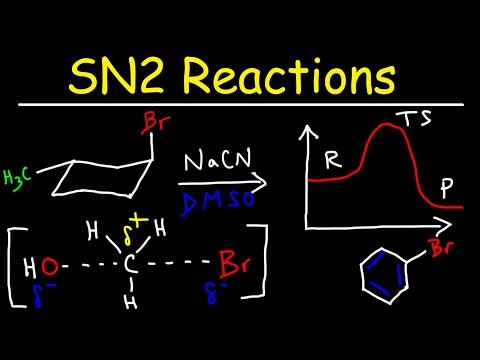 0:22:49
0:22:49
 0:06:05
0:06:05
 0:02:00
0:02:00
 0:29:21
0:29:21
 0:12:32
0:12:32
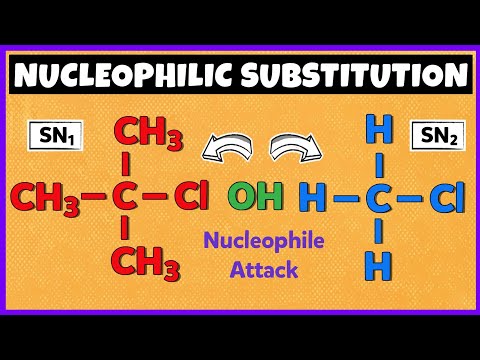 0:08:03
0:08:03
 0:12:19
0:12:19
 0:08:17
0:08:17
 0:29:01
0:29:01
 0:11:31
0:11:31
 0:18:52
0:18:52
 0:07:29
0:07:29
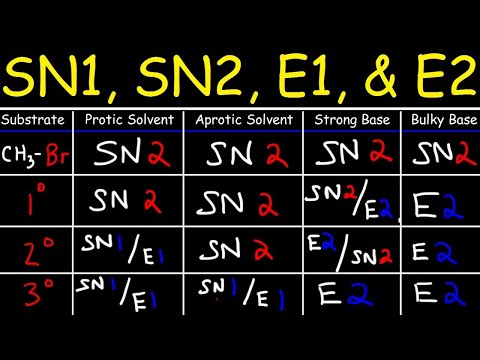 0:38:50
0:38:50
 0:07:50
0:07:50
 1:13:40
1:13:40
 0:16:36
0:16:36
 0:01:00
0:01:00
 0:28:42
0:28:42
 0:00:44
0:00:44
 0:00:31
0:00:31
 0:00:59
0:00:59
 0:09:06
0:09:06
 0:15:16
0:15:16
 0:00:06
0:00:06