filmov
tv
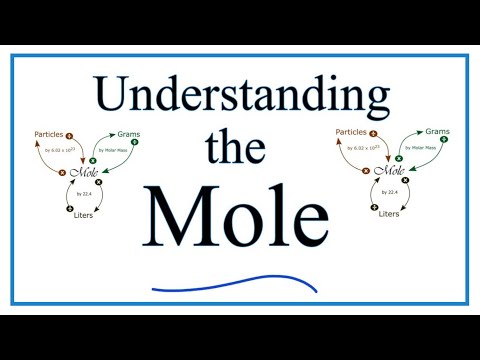
Understanding the Mole (the basics)

Показать описание
The Mole is an essential concept in chemistry. In this video we'll look at how to use the mole to solve chemistry problems found in general chemistry. This includes defining the mole, calculating molar mass, mole ratios, and other key concepts.
For more help with moles to grams conversions and more:
Converting between moles and grams is the cornerstone of being successful in stoichiometry, the study of chemical quantities. Take the time to learn mole conversions and you will find chemistry is much easier.
The use of conversion factors (also called factor-label method or dimensional analysis) is a more general technique for converting quantities. Once you understand how it works it can be applied to many different conversion (as long as you know the conversion factor).
For more help with moles to grams conversions and more:
Converting between moles and grams is the cornerstone of being successful in stoichiometry, the study of chemical quantities. Take the time to learn mole conversions and you will find chemistry is much easier.
The use of conversion factors (also called factor-label method or dimensional analysis) is a more general technique for converting quantities. Once you understand how it works it can be applied to many different conversion (as long as you know the conversion factor).
Understanding the Mole (the basics)
An Actually Good Explanation of Moles
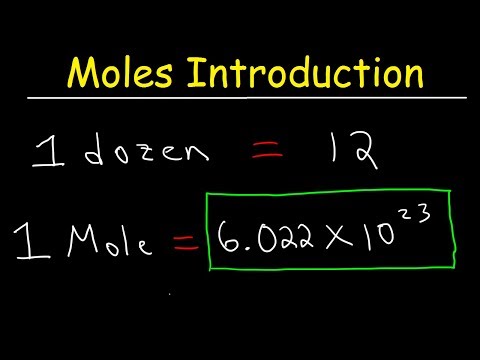
Introduction to Moles
The Mole: Avogadro's Number and Stoichiometry
Concept of Mole - Part 1 | Atoms and Molecules | Infinity Learn
GCSE Chemistry - The Mole (Higher Tier) #25
Mole Concept || 3D Animated explanation || Complete basics || Chemistry|| Class 9, 10, 11, & 12...
Science Raps: GCSE Chemistry - The Mole
Some Basic Concepts of Chemistry 05 | molecular mass, molarity, molality, mole fraction, normality
Mole Calculations Explained!
Mole Concept
MoLE ConCepT in 40 mins : CBSE / ICSE : CHEMISTRY : Class 10, Class 11, Class 12
Stoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice Problems
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6
Mole Concept Class 11 | Chemistry
Relative Formula Mass - mole concept
Mole Conversions Made Easy: How to Convert Between Grams and Moles
GCSE Chemistry - The Mole Explained 💥💥🤯
What Is Avogadro's Number - The Mole | Chemical Calculations | Chemistry | FuseSchool
Mole Concept Important Formulas 💯
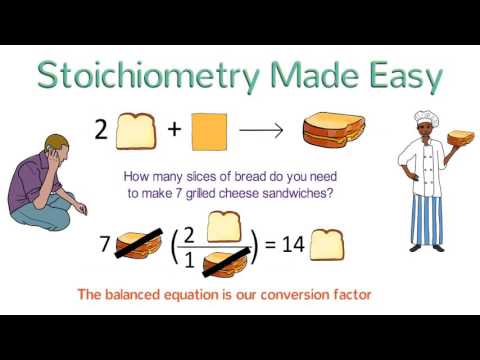
Stoichiometry Made Easy: Stoichiometry Tutorial Part 1
Happy MOLE DAY! 10/23 But what is a mole (mol)? 🤔 #chemistry
What Is Mole ? || Avogadro's Number || Mole Concept || Rajeev Sir || #science #Short #armscaree...
What is Avogadro's Number | Concept of Mole | #avogadro #mole #concept #shorts
Комментарии
 0:03:41
0:03:41
 0:13:37
0:13:37
 0:05:16
0:05:16
 0:06:06
0:06:06
 0:03:09
0:03:09
 0:04:29
0:04:29
 0:02:30
0:02:30
 0:01:07
0:01:07
 0:23:10
0:23:10
 0:00:11
0:00:11
 0:12:23
0:12:23
 0:37:58
0:37:58
 0:25:16
0:25:16
 0:12:47
0:12:47
 0:12:59
0:12:59
 0:05:57
0:05:57
 0:07:25
0:07:25
 0:00:18
0:00:18
 0:04:02
0:04:02
 0:00:14
0:00:14
 0:06:55
0:06:55
 0:00:21
0:00:21
 0:01:00
0:01:00
 0:00:54
0:00:54