filmov
tv
What Is Avogadro's Number - The Mole | Chemical Calculations | Chemistry | FuseSchool

Показать описание
Learn the basics about Avogadro's Number, as a part of chemical calculations.
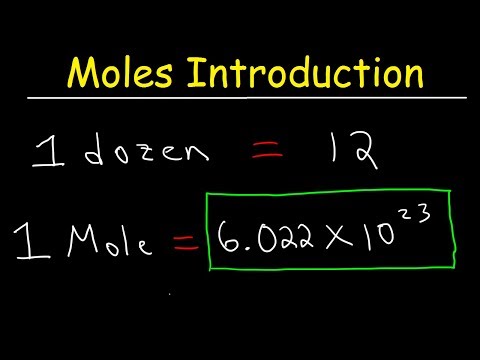
Avogadro’s Number or the Avogadro Constant is 6.02 X 1023 Mol-1. It is the number of atoms per mole of the carbon-12 isotope.
For atoms, the mass of Avogadro’s number of particles is equal to their relative atomic mass in grams.
For molecules, the mass of Avogadro’s number of particles is equal to their relative molecular mass in grams.
What is Molar Volume?
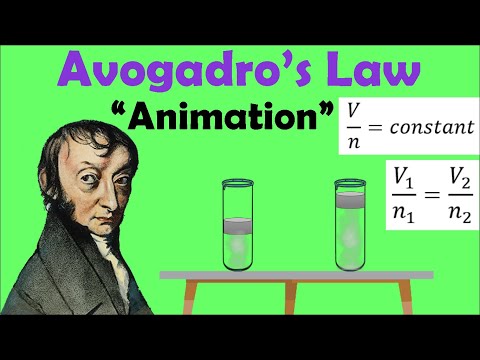
In 1811, Avogadro proposed his hypothesis which stated ‘Equal volumes of gases at the same temperature and pressure contain the same number of molecules’. This hypothesis made it much easier to perform calculations involving gases. So one mole (Avogadro’s number of molecules) of any gas will occupy the same volume, at the same temperature and pressure, as one mole of any other gas.
The volume occupied by one mole of a gas is called the Molar Volume.
Molar volume is measured in dm3mol-1.
SUBSCRIBE to the Fuse School YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
This video is part of 'Chemistry for All' - a Chemistry Education project by our Charity Fuse Foundation - the organisation behind The Fuse School. These videos can be used in a flipped classroom model or as a revision aid. Find our other Chemistry videos here:
Avogadro’s Number or the Avogadro Constant is 6.02 X 1023 Mol-1. It is the number of atoms per mole of the carbon-12 isotope.
For atoms, the mass of Avogadro’s number of particles is equal to their relative atomic mass in grams.
For molecules, the mass of Avogadro’s number of particles is equal to their relative molecular mass in grams.
What is Molar Volume?
In 1811, Avogadro proposed his hypothesis which stated ‘Equal volumes of gases at the same temperature and pressure contain the same number of molecules’. This hypothesis made it much easier to perform calculations involving gases. So one mole (Avogadro’s number of molecules) of any gas will occupy the same volume, at the same temperature and pressure, as one mole of any other gas.
The volume occupied by one mole of a gas is called the Molar Volume.
Molar volume is measured in dm3mol-1.
SUBSCRIBE to the Fuse School YouTube channel for many more educational videos. Our teachers and animators come together to make fun & easy-to-understand videos in Chemistry, Biology, Physics, Maths & ICT.
This video is part of 'Chemistry for All' - a Chemistry Education project by our Charity Fuse Foundation - the organisation behind The Fuse School. These videos can be used in a flipped classroom model or as a revision aid. Find our other Chemistry videos here:
Комментарии
 0:06:06
0:06:06
 0:04:02
0:04:02
 0:04:33
0:04:33
 0:07:29
0:07:29
 0:07:14
0:07:14
 0:04:02
0:04:02
 0:08:57
0:08:57
 0:34:55
0:34:55
 0:02:47
0:02:47
 0:13:37
0:13:37
 0:10:50
0:10:50
 0:06:00
0:06:00
 0:08:21
0:08:21
 0:02:57
0:02:57
 0:10:28
0:10:28
 0:14:27
0:14:27
 0:05:28
0:05:28
 0:10:01
0:10:01
 0:08:57
0:08:57
 0:07:02
0:07:02
 0:09:57
0:09:57
 0:05:16
0:05:16
 0:03:07
0:03:07
 0:03:10
0:03:10