filmov
tv
Mole Concept

Показать описание
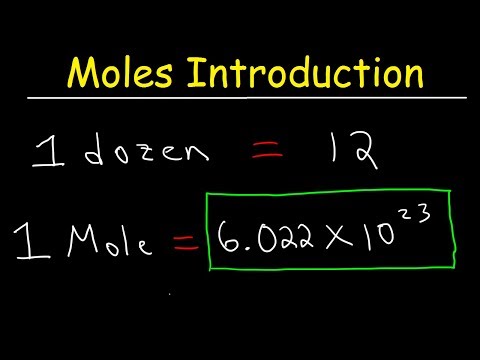
Do you know how much is 1 mole? Mole Concept is explained in terms of mass, count of particles and volume of a gas.
Avogadro's Number also known as Avogadro's Constant is also explained. Mole is a very important unit in Chemistry.
Solutions of Top 3 Questions on Mole Concept: COMING SOON !!
The questions are shown at the end of the video.
At Manocha Academy, learning Science and Math is Easy! The school coursework is explained with simple examples that you experience every day! Yes, Science & Math is all around you! Let's learn every day from everyday life!
Avogadro's Number also known as Avogadro's Constant is also explained. Mole is a very important unit in Chemistry.
Solutions of Top 3 Questions on Mole Concept: COMING SOON !!
The questions are shown at the end of the video.
At Manocha Academy, learning Science and Math is Easy! The school coursework is explained with simple examples that you experience every day! Yes, Science & Math is all around you! Let's learn every day from everyday life!
Introduction to Moles
GCSE Chemistry - The Mole (Higher Tier) #25
Relative Formula Mass - mole concept
Mole Concept
Introduction to Moles
Concept of Mole | Avogadro's Number | Atoms and Molecules | Don't Memorise
Mole Concept Class 11 | Chemistry
Concept of Mole - Part 1 | Atoms and Molecules | Infinity Learn
L-1 Mole Concept By MMD Sir Intelligence Career Institute
MoLE ConCepT in 40 mins : CBSE / ICSE : CHEMISTRY : Class 10, Class 11, Class 12
Plus One Chemistry | Some Basic Concepts of Chemistry |MOLE CONCEPT | Exam Winner
GCE's And G10-12 - Full Mole Concept [Stoichiometric calculations]
SHS 2 - INTEGRATED SCIENCE - THE MOLE CONCEPT
An Actually Good Explanation of Moles
The Mole: Avogadro's Number and Stoichiometry
CSEC Chemistry - The Mole Concept 1
How big is a mole? (Not the animal, the other one.) - Daniel Dulek
Mole Concept | Complete Chapter | GAME of NEET | Wassim
MOLE Concept in 6 mins : Class X CBSE / ICSE :
Mole Concept || 3D Animated explanation || Complete basics || Chemistry|| Class 9, 10, 11, & 12...
Mole Concept Full Marathon | Class 11 Chemistry | NEET 2024 |🔥Shimon sir
Mole Concept | Chemistry
Relative Atomic Mass (Ar) - mole concept
O-Level Chemistry | 11 | Mole Concept [1/4]
Комментарии
 0:05:16
0:05:16
 0:04:29
0:04:29
 0:05:57
0:05:57
 0:12:23
0:12:23
 0:10:50
0:10:50
 0:06:00
0:06:00
 0:12:59
0:12:59
 0:03:09
0:03:09
 0:27:04
0:27:04
 0:37:58
0:37:58
 0:23:03
0:23:03
 0:55:49
0:55:49
 0:57:23
0:57:23
 0:13:37
0:13:37
 0:06:06
0:06:06
 0:20:50
0:20:50
 0:04:33
0:04:33
 8:39:44
8:39:44
 0:06:17
0:06:17
 0:02:30
0:02:30
 3:01:04
3:01:04
 0:13:16
0:13:16
 0:05:32
0:05:32
 0:15:05
0:15:05