filmov
tv
Shells, Sub-shells, and Orbitals l Understand the difference

Показать описание
It requires energy to take an electron away from the nucleus.
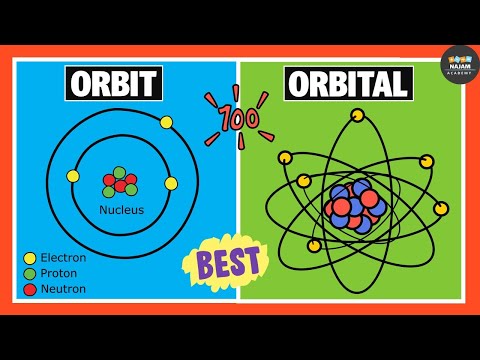
The circular path near the nucleus has lower energy than the one that is farther away from it.
These circular paths are called shells or energy levels.
The energy levels or the shells are represented K, L, M, and N shells.
The number of maximum electrons in any shell can be calculated by using the general formula 2n²
where n represents the energy level.
For example, when n=2, the number of electrons in the 2nd energy level are 2x2² = 2x4 = 8 electrons.
Each has sub-shells.
The first energy level has 1 sub-shell called 's' sub-shell
The second shell has 2 sub-shells: s and p
The third energy level has 3 sub-shells: s, p, d
The fourth energy level or the shell has 4 sub-shells: s, p, d, f
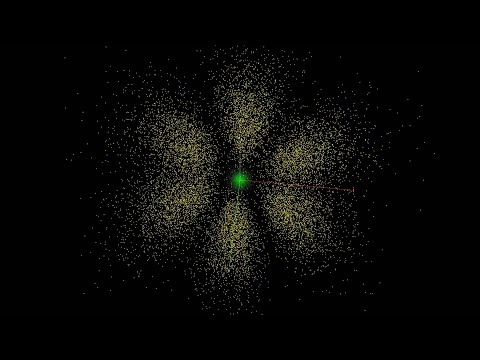
Within sub-shells, we have orbitals, the final location of the electrons.
s subshell has 1 orbital
p has 3 orbitals
d has 5 orbitals
and
f has 7 orbitals
Each orbital can accommodate a maximum of 2 electrons.
In short,
In shells we have sub-shells, and within subshells we see orbitals.
#shells #subshells #orbitals
Thank you for watching the video.
Kindly subscribe to the channel for more interesting updates.
Yash :)
The circular path near the nucleus has lower energy than the one that is farther away from it.
These circular paths are called shells or energy levels.
The energy levels or the shells are represented K, L, M, and N shells.
The number of maximum electrons in any shell can be calculated by using the general formula 2n²
where n represents the energy level.
For example, when n=2, the number of electrons in the 2nd energy level are 2x2² = 2x4 = 8 electrons.
Each has sub-shells.
The first energy level has 1 sub-shell called 's' sub-shell
The second shell has 2 sub-shells: s and p
The third energy level has 3 sub-shells: s, p, d
The fourth energy level or the shell has 4 sub-shells: s, p, d, f
Within sub-shells, we have orbitals, the final location of the electrons.
s subshell has 1 orbital
p has 3 orbitals
d has 5 orbitals
and
f has 7 orbitals
Each orbital can accommodate a maximum of 2 electrons.
In short,
In shells we have sub-shells, and within subshells we see orbitals.
#shells #subshells #orbitals
Thank you for watching the video.
Kindly subscribe to the channel for more interesting updates.
Yash :)
Комментарии
 0:09:23
0:09:23
 0:06:00
0:06:00
 0:11:19
0:11:19
 0:08:42
0:08:42
 0:14:27
0:14:27
 0:20:21
0:20:21
 0:03:25
0:03:25
 0:13:04
0:13:04
 0:05:05
0:05:05
 0:03:24
0:03:24
 0:02:23
0:02:23
 0:03:50
0:03:50
 0:06:55
0:06:55
 0:09:43
0:09:43
 0:05:50
0:05:50
 0:21:34
0:21:34
 0:08:11
0:08:11
 0:03:46
0:03:46
 0:02:09
0:02:09
 0:00:15
0:00:15
 0:10:05
0:10:05
 0:01:40
0:01:40
 0:10:13
0:10:13
 0:00:16
0:00:16