filmov
tv
What are: Shells, Subshells, Orbitals, Pauli and Quantum Numbers

Показать описание
"Doers and Thinkers" - Because that's what we are...
Brief video explaining the relationship between the electron configuration and quantum numbers. Also attempt to explain shell, subshell and orbital in simple terms. Thanks for watching.
My goal with "Doers and Thinkers" is to help you get the answer your looking for as quickly as possible. So that you may get back to DOING and THINKING the worlds greatest innovations.
Brief video explaining the relationship between the electron configuration and quantum numbers. Also attempt to explain shell, subshell and orbital in simple terms. Thanks for watching.
My goal with "Doers and Thinkers" is to help you get the answer your looking for as quickly as possible. So that you may get back to DOING and THINKING the worlds greatest innovations.
What are Shells, Subshells, and Orbitals? | Chemistry
Shells, subshells, and orbitals | Atomic structure and properties | AP Chemistry | Khan Academy
Shells, Subshells, and Orbitals - BIOLOGY/CHEMISTRY EP5
Shells-Subshells-Orbitals
Elements, Atoms, Shells, Subshells And Orbitals
Shells, subshells and orbitals #chemistrywithsoumya #orbitals #subshells #orbits #shells
Relationship of Quantum shells, Subshells, Orbitals & Electrons EXPLAINED [GCE A Level Chemistry...
Orbitals, Atomic Energy Levels, & Sublevels Explained - Basic Introduction to Quantum Numbers
Orbitals: Crash Course Chemistry #25
Electron Configuration - Electron Subshells - Suborbitals - s, p, d, f - Orbitals - Chemistry
What are shells,subshells and orbitals?| Difference between shells, subshells and orbitals|chemistry
Chemistry 101: Shells, Subshells, and Atomic Orbitals
What ARE atomic orbitals?
electron configuration #shell#subshell#orbitals
Inside Atoms: Electron Shells and Valence Electron
A Better Way To Picture Atoms
Shells, Sub-shells, and Orbitals l Understand the difference
Quantum Numbers, Atomic Orbitals, and Electron Configurations
Are Subshells and Orbitals same ? | Shell subshell orbital #neet #class11chemistry #shorts
What are: Shells, Subshells, Orbitals, Pauli and Quantum Numbers
Atomic orbitals explained #shorts #science
number of electrons in shells|electron distribution in shells|electron orbitals|k l m n shells#short
What is Shell, Subshell and Orbital | Class 11, IIT-JEE & NEET | by Sarvesh Sir
Atomic orbitals 3D
Комментарии
 0:06:00
0:06:00
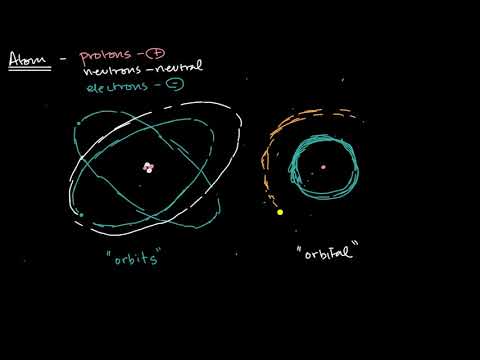 0:09:41
0:09:41
 0:09:23
0:09:23
 0:03:23
0:03:23
 0:14:27
0:14:27
 0:00:59
0:00:59
 0:05:05
0:05:05
 0:11:19
0:11:19
 0:10:52
0:10:52
 0:20:21
0:20:21
 0:12:30
0:12:30
 0:04:36
0:04:36
 0:21:34
0:21:34
 0:00:16
0:00:16
 0:03:25
0:03:25
 0:05:35
0:05:35
 0:13:04
0:13:04
 0:08:42
0:08:42
 0:01:00
0:01:00
 0:06:55
0:06:55
 0:00:16
0:00:16
 0:00:09
0:00:09
 0:05:50
0:05:50
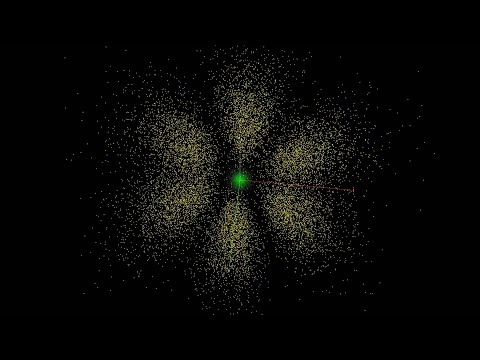 0:05:50
0:05:50