filmov
tv
Ionic compounds | “Dot and cross” diagrams | A Level H2 Chem | Making Sense Chem

Показать описание
In today’s video, we will be talking about ionic bonds!
music by:
0:00 Introduction
1:50 What is an Ionic bond?
4:10 Drawing "dot and cross" for ionic compounds
5:20 Lattice structure of ionic compounds
8:00 Strength of ionic bonds
2 types of bonding:
1) lattice bonds (strong forces)
ionic
giant covalent
metallic
2) Intermolecular attractions (weak forces).
instantaneous dipole - induced dipole (id-id)
permanent dipole - permanent dipole (pd-pd)
hydrogen bonds (h-bond)
Metals lose electrons while non-metals gain electrons.
Ionic bond: electrostatic forces of attraction between cations and anions.
Ionic bonding involves electron transfer from a metal to a non-metal element to form a stable species.
Ionic bonding occurs between elements with large electronegativity difference.
Electronegativity: tendency to attract bonding electrons toward itself
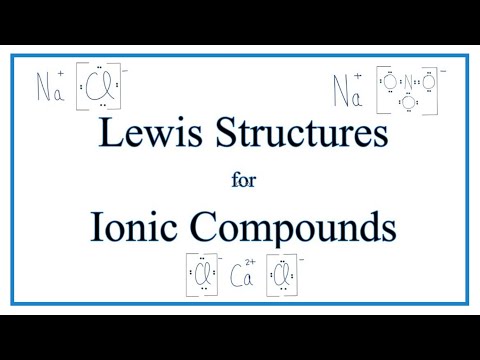
"Dot and cross" diagrams:
1) Don't draw valence electrons of metals once it loses its valence electrons
2) Don't draw the shells for each atoms
Lattice structure of ionic compounds:
Ions are arranged in an alternative manner to maximise attractive forces and minimise repulsion.
Ionic bond is non-directional: an ion attracts oppositely charged ion equally in all directions.
Strength of ionic bonds:
Lattice energy, LE: energy released when one mole of solid ionic compound is formed from its constituent gaseous ions under standard conditions of 298K and 1 bar.
Mg²⁺(g) + 2 Cl⁻(g) → MgCl(s)
Larger lattice energy = stronger ionic bond
Bonds are formed when cations and anions are attracted to each other hence all lattice energies are exothermic.
LE is proportional to product of charges
LE is inversely proportional to sum of ionic radius
Larger ionic charge = stronger LE = stronger ionic bonds
Smaller ionic radius = stronger LE = stronger ionic bonds
Which factor (ionic charge or ionic radius) is more important in explaining magnitude of LE?
Charges are multiplied, radiuses are added together.
Therefore, multiplication has a larger effect on LE.
Follow us on:
Youtube - Making Sense Tuition Centre
Tik tok - @makingsensechemistry
Instagram - @makingsensechemistry
Facebook - Making Sense, Singapore's Leading Chemistry Tuition
The Best Chemistry tuition!
#indigoeducationgroup
#bestchemistrytuition
#makingsensechemistry
#learningisfun
#videolessons
#educationalvideos
#easyandfunlearning
#chemicalbonding
#ionicbonds
music by:
0:00 Introduction
1:50 What is an Ionic bond?
4:10 Drawing "dot and cross" for ionic compounds
5:20 Lattice structure of ionic compounds
8:00 Strength of ionic bonds
2 types of bonding:
1) lattice bonds (strong forces)
ionic
giant covalent
metallic
2) Intermolecular attractions (weak forces).
instantaneous dipole - induced dipole (id-id)
permanent dipole - permanent dipole (pd-pd)
hydrogen bonds (h-bond)
Metals lose electrons while non-metals gain electrons.
Ionic bond: electrostatic forces of attraction between cations and anions.
Ionic bonding involves electron transfer from a metal to a non-metal element to form a stable species.
Ionic bonding occurs between elements with large electronegativity difference.
Electronegativity: tendency to attract bonding electrons toward itself
"Dot and cross" diagrams:
1) Don't draw valence electrons of metals once it loses its valence electrons
2) Don't draw the shells for each atoms
Lattice structure of ionic compounds:
Ions are arranged in an alternative manner to maximise attractive forces and minimise repulsion.
Ionic bond is non-directional: an ion attracts oppositely charged ion equally in all directions.
Strength of ionic bonds:
Lattice energy, LE: energy released when one mole of solid ionic compound is formed from its constituent gaseous ions under standard conditions of 298K and 1 bar.
Mg²⁺(g) + 2 Cl⁻(g) → MgCl(s)
Larger lattice energy = stronger ionic bond
Bonds are formed when cations and anions are attracted to each other hence all lattice energies are exothermic.
LE is proportional to product of charges
LE is inversely proportional to sum of ionic radius
Larger ionic charge = stronger LE = stronger ionic bonds
Smaller ionic radius = stronger LE = stronger ionic bonds
Which factor (ionic charge or ionic radius) is more important in explaining magnitude of LE?
Charges are multiplied, radiuses are added together.
Therefore, multiplication has a larger effect on LE.
Follow us on:
Youtube - Making Sense Tuition Centre
Tik tok - @makingsensechemistry
Instagram - @makingsensechemistry
Facebook - Making Sense, Singapore's Leading Chemistry Tuition
The Best Chemistry tuition!
#indigoeducationgroup
#bestchemistrytuition
#makingsensechemistry
#learningisfun
#videolessons
#educationalvideos
#easyandfunlearning
#chemicalbonding
#ionicbonds
 0:04:12
0:04:12
 0:06:23
0:06:23
 0:05:42
0:05:42
 0:07:20
0:07:20
 0:02:55
0:02:55
 0:06:12
0:06:12
 0:04:10
0:04:10
 0:03:33
0:03:33
 0:08:40
0:08:40
 0:04:44
0:04:44
 0:05:15
0:05:15
 0:15:08
0:15:08
 0:04:45
0:04:45
 0:04:41
0:04:41
 0:11:50
0:11:50
 0:02:01
0:02:01
 0:01:47
0:01:47
 0:04:05
0:04:05
 0:08:41
0:08:41
 0:05:07
0:05:07
 0:13:33
0:13:33
 0:47:35
0:47:35
 0:02:15
0:02:15
 0:05:33
0:05:33