filmov
tv
16.1 Introducing pH scale

Показать описание
A. General concept
All aqueous solutions contain H2O, therefore, they contains H+ and OH-
Acidity and alkalinity depends on the difference between [H+] and [OH-]
If [H+] larger than [OH-] : Acidic
[H+] = [OH-] : Neutral
[H+]smaller than [OH-] : Alkaline
B. Calculating pH value
pH = -log [H+]
pH has no unit!
[ ] means molar concentration, with the unit M
When pH decreases by 1 (more acidic), [H+] increases 10 times ~!!! Because of log ~!
**Pay attention to calculating pH of polybasic acid :
e.g.
2M H3PO4 : pH = -log [2 x 3 ] due to each molecule produces 3 H+ ion. ~!!
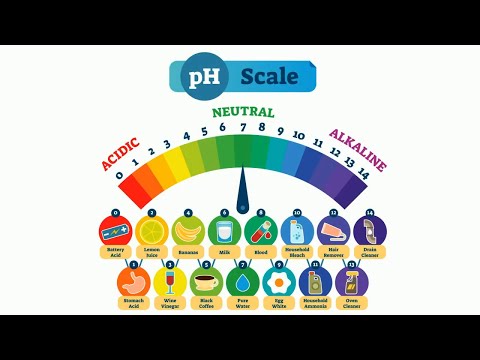
C. pH Scale
Range from 0 to 14. But we have solution smaller than 0 or larger than 14.
Recognize the pH value of some daily commodity.
e.g.
Alkaline: Window clearner, drain clearner, soap, baking soda
Acidic: orange juice, coke, stomach juice, car battery
All aqueous solutions contain H2O, therefore, they contains H+ and OH-
Acidity and alkalinity depends on the difference between [H+] and [OH-]
If [H+] larger than [OH-] : Acidic
[H+] = [OH-] : Neutral
[H+]smaller than [OH-] : Alkaline
B. Calculating pH value
pH = -log [H+]
pH has no unit!
[ ] means molar concentration, with the unit M
When pH decreases by 1 (more acidic), [H+] increases 10 times ~!!! Because of log ~!
**Pay attention to calculating pH of polybasic acid :
e.g.
2M H3PO4 : pH = -log [2 x 3 ] due to each molecule produces 3 H+ ion. ~!!
C. pH Scale
Range from 0 to 14. But we have solution smaller than 0 or larger than 14.
Recognize the pH value of some daily commodity.
e.g.
Alkaline: Window clearner, drain clearner, soap, baking soda
Acidic: orange juice, coke, stomach juice, car battery
 0:18:30
0:18:30
 0:03:09
0:03:09
 0:04:37
0:04:37
 0:27:02
0:27:02
 0:58:42
0:58:42
 0:04:54
0:04:54
 0:10:35
0:10:35
 0:16:05
0:16:05
 0:07:45
0:07:45
 0:05:34
0:05:34
 0:07:06
0:07:06
 0:16:04
0:16:04
 0:03:10
0:03:10
 0:09:01
0:09:01
 0:00:31
0:00:31
 0:05:55
0:05:55
 0:24:29
0:24:29
 0:03:17
0:03:17
 0:03:16
0:03:16
 0:07:07
0:07:07
 0:00:30
0:00:30
 0:18:52
0:18:52
 0:00:41
0:00:41
 0:00:26
0:00:26