filmov
tv
Molarity of a Diluted Solution

Показать описание
Chapter 12 Slide 76 Example Calculation
What is the molarity of a solution when 75.0 mL of a 4.00 M KCl solution is diluted to a volume of 500. mL?
What is the molarity of a solution when 75.0 mL of a 4.00 M KCl solution is diluted to a volume of 500. mL?
Dilution Problems, Chemistry, Molarity & Concentration Examples, Formula & Equations
Molarity and Dilution
Molarity of a Diluted Solution
Molarity Made Easy: How to Calculate Molarity and Make Solutions
Dilution Problems - Chemistry Tutorial
Molarity Practice Problems
4.4 Molarity and Dilutions | General Chemistry
How to Dilute a Solution
The Simple Way To Solve Hard Molarity Calculations
How to Use the Dilution Equation
How to calculate molarity from titration data? | Stock Solution vs Diluted Solution
Molarity, Solution Stoichiometry and Dilution Problem
Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density - Solution Concentration ...
The C1V1 = C2V2 Equation Explained
Concentration and Molarity explained: what is it, how is it used + practice problems
3.63a | Find the molarity: 1.00 L of a 0.250-M solution of Fe(NO3)3 is diluted to a final volume of
Calculating Molarity | Molar Concentration #chemistry #science #homework #shorts #short #education
30 mL of 0.5 M H2SO4 diluted to 500 mL. Calculate molarity.
What is Dilute Solution? Chemistry
DILUTIONS Calculate the molarity of the diluted solution 1 3.3 M 85.0 mL 15.0 mL TYPE
Dilute or Concentrated Acids/Bases | Don't Memorise
Dilution | Intermolecular forces and properties | AP Chemistry | Khan Academy
Molarity and Dilution Practice Problems
DILUTION OF A SOLUTION [ M1V1 = M2V2 ] || SOLUTION & COLLIGATIVE PROPERTIES - 11
Комментарии
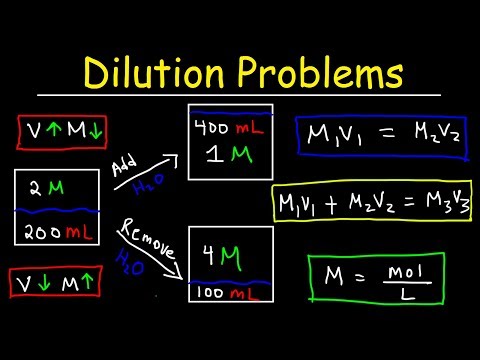 0:21:55
0:21:55
 0:02:30
0:02:30
 0:02:35
0:02:35
 0:08:46
0:08:46
 0:06:14
0:06:14
 0:21:27
0:21:27
 0:16:12
0:16:12
 0:03:36
0:03:36
 0:14:15
0:14:15
 0:10:35
0:10:35
 0:06:12
0:06:12
 0:10:25
0:10:25
 0:31:25
0:31:25
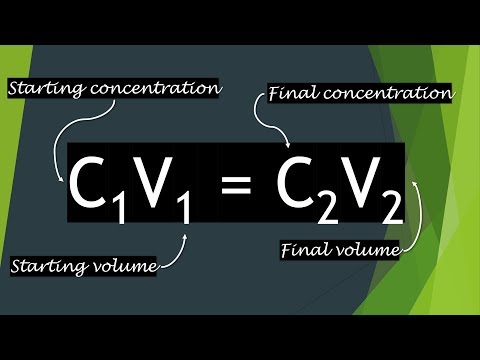 0:05:27
0:05:27
 0:05:41
0:05:41
 0:03:30
0:03:30
 0:00:58
0:00:58
 0:00:59
0:00:59
 0:05:04
0:05:04
 0:00:33
0:00:33
 0:03:20
0:03:20
 0:05:53
0:05:53
 0:15:04
0:15:04
 0:15:31
0:15:31