filmov
tv
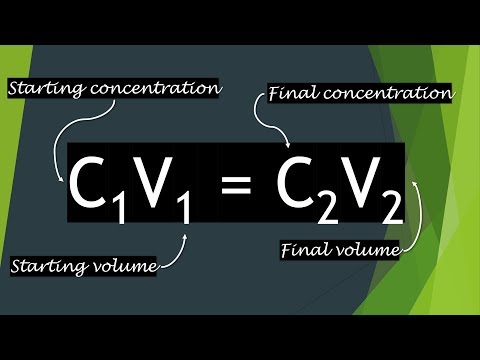
The C1V1 = C2V2 Equation Explained

Показать описание
The simple formula of C1V1 = C2V2 is a lifesaver for bioscience researchers in the lab who are wanting to do dilutions. Here I will explain what the equation means and how you can use it.
THE ONLINE GUIDE
VIDEO BREAKDOWN
Step 1: Equation overview (00:14)
Step 2: Calculating C1 (01:07)
Step 3: Calculating V2 (01:39)
Step 4: Example 1 (01:49)
Step 5: Example 2 (03:32)
MORE HELPFUL HINTS & TIPS
FOLLOW US
THE ONLINE GUIDE
VIDEO BREAKDOWN
Step 1: Equation overview (00:14)
Step 2: Calculating C1 (01:07)
Step 3: Calculating V2 (01:39)
Step 4: Example 1 (01:49)
Step 5: Example 2 (03:32)
MORE HELPFUL HINTS & TIPS
FOLLOW US
The C1V1 = C2V2 Equation Explained
C1V1 = C2V2 Practice Problem #chemistry #dilution
How to Use the Dilution Equation
Dilution Calculation Practice (C1V1 = C2V2)
How to use Dilution Equation C1V1 = C2V2
Dilution Problems, Chemistry, Molarity & Concentration Examples, Formula & Equations
✨Fundamental lab skills part ✌🏼✨ C1V1=C2V2 calculating dilutions. #scienceexperiment #phd #phdlife...
Using the Dilution Formula (C1V1=C2V2)
Calculation on C1V1=C2V2 formula
Dilution Calculations: C1V1 = C2V2 (Moles Unit)
Dilution practice problem, equation explanation C1V1=C2V2
Dilution in biology lab: How to use the equation C1V1 = C2V2
Dilutions or C1V1 = C2V2
Dilution Equation
Easy DILUTIONS (C1V1=C2V2)
Making Dilutions with M1V1 = M2V2 #chemistry #stemeducation #edushorts #stem
C1V1 = C2V2 (aka M1V1=M2V2) is so useful for calculating solution volumes & concentrations!
Dilutions C1V1 = C2V2
C1V1=C2V2; concentration-calculating & molarity hilarity
D'où provient C1V1 = C2V2 ?
Video 18 Simple C1V1 = C2V2 dilution calculations
Dilution calculations | Dilution problems | Stock dilutions Biology and chemistry |
Calculating Molarity using C1V1=C2V2 and C=n/L
Dilution Calculation
Комментарии
 0:05:27
0:05:27
 0:00:58
0:00:58
 0:10:35
0:10:35
 0:07:34
0:07:34
 0:07:49
0:07:49
 0:21:55
0:21:55
 0:00:53
0:00:53
 0:18:58
0:18:58
 0:04:41
0:04:41
 0:08:59
0:08:59
 0:04:08
0:04:08
 0:02:54
0:02:54
 0:02:41
0:02:41
 0:07:55
0:07:55
 0:06:05
0:06:05
 0:00:58
0:00:58
 0:13:34
0:13:34
 0:07:34
0:07:34
 0:18:24
0:18:24
 0:08:00
0:08:00
 0:07:00
0:07:00
 0:19:36
0:19:36
 0:06:05
0:06:05
 0:02:26
0:02:26