filmov
tv
Use the rate law in Example Problem 16.2 and the concentrations given in Practice Problems 31 and 3…

Показать описание
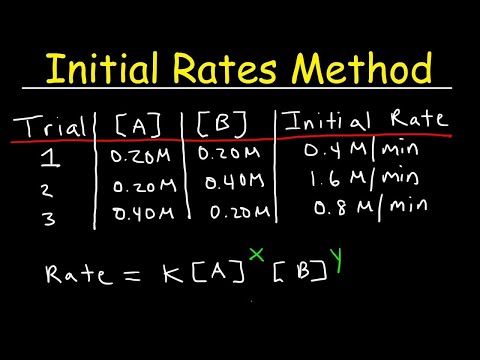
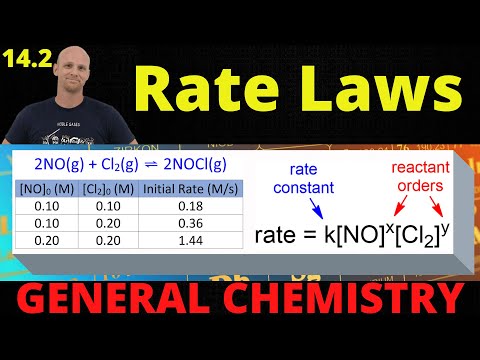
Use the rate law in Example Problem 16.2 and the concentrations given in Practice Problems 31 and 32 to calculate the instantaneous rate for the reaction between NO and H_2 . Challenge Calculate [NO] for the reaction in Example Problem 16.2 if the rate is 9.00 ×10^-5 mol /(L ·s) and [H_2] is 0.00300 M .
Watch the full video at:
Never get lost on homework again. Numerade is a STEM learning website and app with the world’s largest STEM video library.
Join today and access millions of expert-created videos, each one skillfully crafted to teach you how to solve tough problems step-by-step.
Join Numerade today at:
Watch the full video at:
Never get lost on homework again. Numerade is a STEM learning website and app with the world’s largest STEM video library.
Join today and access millions of expert-created videos, each one skillfully crafted to teach you how to solve tough problems step-by-step.
Join Numerade today at:
 0:09:10
0:09:10
 0:03:42
0:03:42
 0:08:44
0:08:44
 0:18:48
0:18:48
 0:01:58
0:01:58
 0:04:04
0:04:04
 0:06:08
0:06:08
 0:34:53
0:34:53
 0:11:27
0:11:27
 0:10:49
0:10:49
 0:25:16
0:25:16
 0:48:46
0:48:46
 0:07:46
0:07:46
 0:12:28
0:12:28
 0:08:42
0:08:42
 0:01:30
0:01:30
 0:05:56
0:05:56
 0:02:22
0:02:22
 0:03:14
0:03:14
 0:10:39
0:10:39
 0:09:00
0:09:00
 0:08:24
0:08:24
 0:08:12
0:08:12
 0:00:41
0:00:41