filmov
tv
Simple and easy science experiments.Electrolysis of water with NaCl.#shorts #diy #experiment #dwe .

Показать описание
Simple and easy science experiments.Electrolysis of water with NaCl.#shorts #diy #experiment #dwe .
Electrolysis Of Water | How To Produce Hydrogen From Water | Water Electrolysis #shorts
water electrolysis experiment ||simple science experiment with water ||easy experiment #shorts
Balloon in a Bottle Science Trick
SCIENCE EXPERIMENTS: easy science experiments To do at home ll #shorts#viral
The Levitating Water Experiment.... What's the secret? #water #science #isitice
Electrolysis using salt experiment.
GRAPHITE + WATER + Battery = SCIENCE EXPERIMENT 🤯🤯#electrolysis #graphite
Electrolysis of Water #shorts
How to Burn Water 🔥💦🧪 #shorts #chemistry #chemicalreaction #magic
Easy simple science experiments for school students| DIY | water salt lemon sinking check|Mr MAK
Reverse Reality With Refraction In This Easy Science Experiment ➡️👀⬅️
Electrolysis of Water Experiment at Home | Science Experiment for Kids | #ExperimentShorts
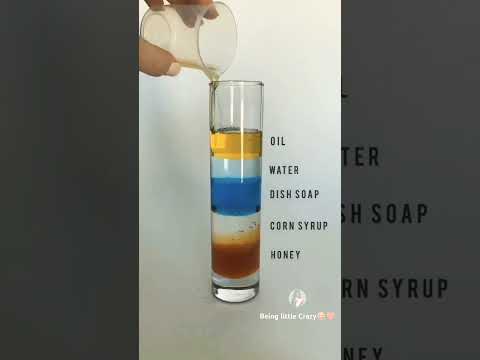
| colourful liquid density gradient | layers of liquid in glass |Awesome science experiment
Simple Science Experiment with Batteries and Salt Water 🤯FAKE or REAL #shorts #experiment
Water vs Battery || H2 and O2 out from water || Water Electrolysis Gas #science #experiment
🔥fevicol vs cool+normal water||experiment|Easy experiment #shorts
Chemical Reaction 🧫🔥।। Easy science experiment 👨🔬।। #ytshorts #viral #shorts #science...
ELECTROLYSIS: Easy Science Experiments To Do At Home, Science Experiments, DIY ideas, DIY, #Shorts
Water Electrolysis Project | school science project | simple experiments #shorts #experiment
Electrolysis in Normal water vs Salt water | electrolysis experiment | school project #experiment
Electrolysis Science Experiment with salt water
Water electrolysis experiment 😱||Simple science experiment with water || Easy Experiment #shorts
🔎🔭Simple Science Experiments | Glowing Bulb From Salt Water #shorts #viral #trending #experiment...
Комментарии
 0:00:15
0:00:15
 0:00:25
0:00:25
 0:00:14
0:00:14
 0:00:17
0:00:17
 0:00:31
0:00:31
 0:00:25
0:00:25
 0:00:43
0:00:43
 0:00:52
0:00:52
 0:01:00
0:01:00
 0:00:22
0:00:22
 0:00:16
0:00:16
 0:00:18
0:00:18
 0:00:57
0:00:57
 0:00:16
0:00:16
 0:00:21
0:00:21
 0:00:54
0:00:54
 0:00:34
0:00:34
 0:00:23
0:00:23
 0:00:59
0:00:59
 0:00:15
0:00:15
 0:00:17
0:00:17
 0:01:00
0:01:00
 0:00:27
0:00:27
 0:00:17
0:00:17