filmov
tv
Determining Molar Solubility Given Ksp
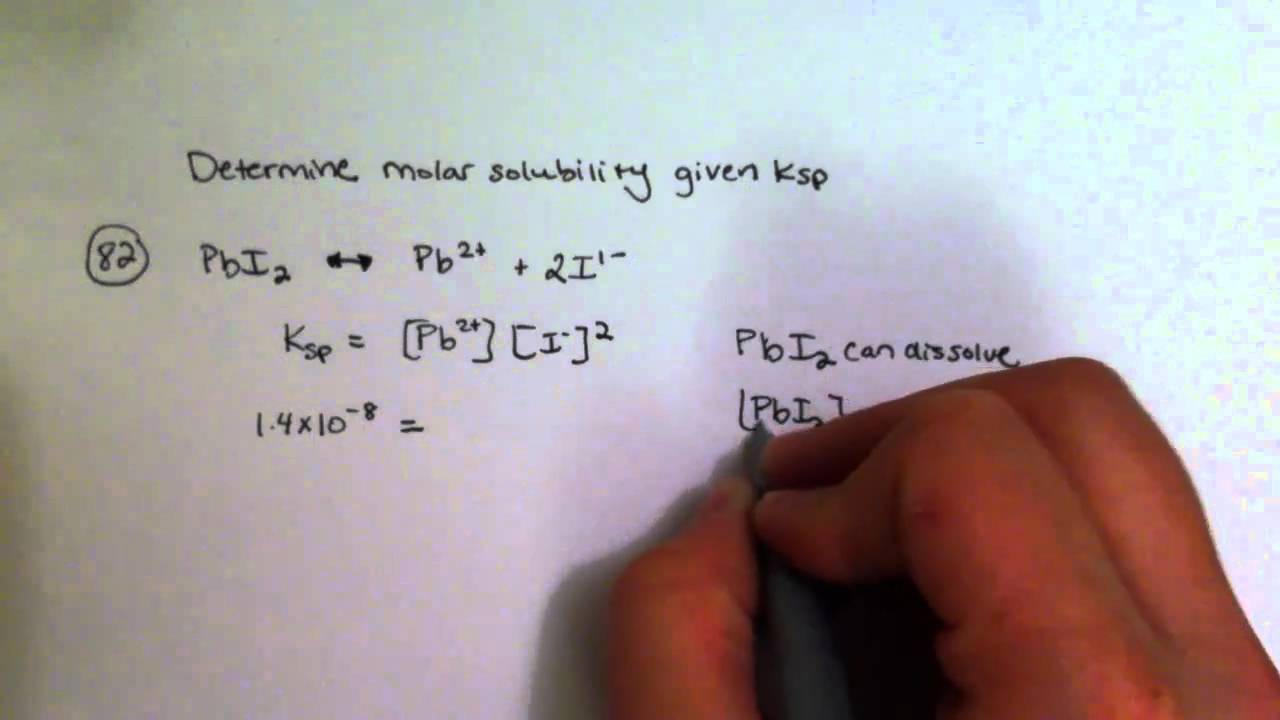
Показать описание
Determining Molar Solubility Given Ksp
Worked example: Calculating solubility from Kₛₚ | Equilibrium | AP Chemistry | Khan Academy
Ksp - Molar Solubility, Ice Tables, & Common Ion Effect
[SHORTCUT] How to Solve for Molar Solubility Given Ksp
4.5.3 - Calculating Molar Solubility from Ksp
How to Solve for Molar Solubility Given Ksp (All Work Shown) Practice Problems & Examples
Determining Molar Solubility Given Ksp
Molar Solubility of PbI2 in 0.10 M NaI solution
Determining Molar Solubility from Ksp Example Calculation
Solubility Product Constant (Ksp)
How to Calculate Molar Solubility from Ksp
Example: Calculating Molar Solubility from Equilibrium Constant (Solubility Equilibrium #2)
37: Calculating solubility, molar solubility, and Ksp
Ksp video 1 Calculating Ksp from Molar Solubility Data for AP Chemistry
Calculating Molar Solubility from Ksp-Practice Problems
Ksp and molar solubility calculations video
Calculate Molar Solubility of PbCl2 From Ksp 004
Solubility | Molar Solubility and Solubility Product (Ksp) with Worked Example Problem!
How to Solve for Ksp Given Molar Solubility (Shortcut and Full Process) Practice Problems, Examples
⚗️ Calculating Molar Solubility in the Presence of a Common Ion
Determine Ksp Given Solubility
17.4 Solubility and Ksp | General Chemistry
⚗️ Calculating Molar Solubility from Kₛₚ (Question 1)
How to Determine Ksp Given Molar Solubility
Комментарии
 0:04:26
0:04:26
 0:04:52
0:04:52
 0:41:52
0:41:52
![[SHORTCUT] How to](https://i.ytimg.com/vi/JKnSfMtykkQ/hqdefault.jpg) 0:04:51
0:04:51
 0:09:11
0:09:11
 0:05:52
0:05:52
 0:08:39
0:08:39
 0:04:45
0:04:45
 0:08:25
0:08:25
 0:08:36
0:08:36
 0:01:38
0:01:38
 0:05:47
0:05:47
 0:10:27
0:10:27
 0:08:47
0:08:47
 0:11:34
0:11:34
 0:08:20
0:08:20
 0:05:58
0:05:58
 0:09:07
0:09:07
 0:05:48
0:05:48
 0:05:45
0:05:45
 0:05:26
0:05:26
 0:22:41
0:22:41
 0:04:06
0:04:06
 0:07:54
0:07:54