filmov
tv
17.4 Solubility and Ksp | General Chemistry

Показать описание
Chad provides an introduction to solubility equilibria with a comprehensive lesson on Solubility and Ksp. This begins with an explanation of how Ksp, the solubility product constant, is the equilibrium constant for a reaction in which a solid salt dissociates into aqueous ions. It is demonstrated how to determine the reaction for any salt as well as how to write the equilibrium constant expression.
The molar solubility is also defined as the molar concentration of salt that dissolves in water. The relationship between Ksp and the molar solubility is determined for a variety of salts, and it is shown how to calculate the molar solubility from the Ksp and the Ksp from the molar solubility.
00:00 Lesson Introduction
00:25 How to Calculate Molar Solubility from Ksp for AgCl
06:07 How to Calculate Molar Solubility from Ksp for Ag2S
11:36 How to Calculate Ksp from Molar Solubility for BiI3
16:19 How to Determine the Most Soluble Compound from Ksp
The molar solubility is also defined as the molar concentration of salt that dissolves in water. The relationship between Ksp and the molar solubility is determined for a variety of salts, and it is shown how to calculate the molar solubility from the Ksp and the Ksp from the molar solubility.
00:00 Lesson Introduction
00:25 How to Calculate Molar Solubility from Ksp for AgCl
06:07 How to Calculate Molar Solubility from Ksp for Ag2S
11:36 How to Calculate Ksp from Molar Solubility for BiI3
16:19 How to Determine the Most Soluble Compound from Ksp
Ksp - Molar Solubility, Ice Tables, & Common Ion Effect
Chapter 17. Creating ICE Tables for Solubility Equilibria and Ksp Equations
Worked example: Calculating solubility from Kₛₚ | Equilibrium | AP Chemistry | Khan Academy
Introduction to Solubility Equilibrium (Ksp) (U17 E1)
Solubility Product (Ksp) Sample Problem: Chapter 17 – Part 11
17 4 Ksp
4 M3 106 Solubility and Ksp
calculations with solubility from Ksp
Solubility, Molar Solubility, and Ksp - AP Chemistry Complete Course - Lesson 23.1
Chapter 17 Solubility and Ksp
Ch 17 Ksp molar solubility & Q Part 4
Another Solubility Product (Ksp) Sample Problem: Chapter 17 – Part 12
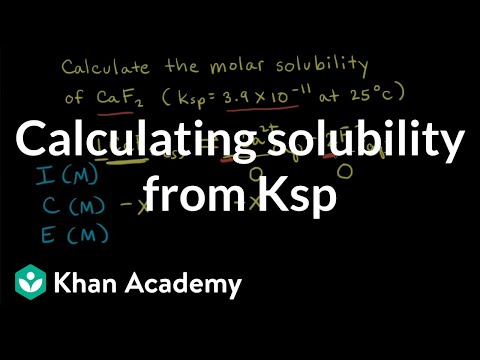
Molar solubility from Ksp
Solubility Product Constant (Ksp): Chapter 17 – Part 3
Use the given molar solubilities in pure water to calculate Ksp for each compound a BaCrO4; molar
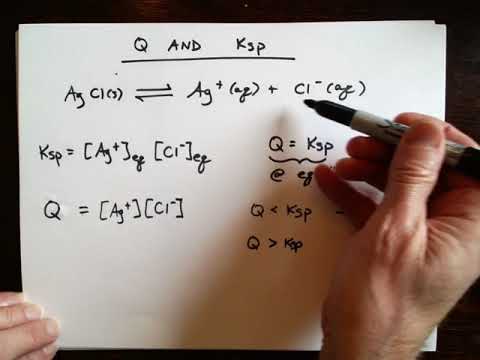
Chapter 17: Q, Ksp, and precipitation
Yet Another Solubility Product (Ksp) Sample Problem: Chapter 17 – Part 14
Common Ion Effect, Ksp, and Solubility. How to Calculate Solubility with Common Ion Effect.
Refer to the Ksp value from Table 17 2 to calculate the solubility of ironII hydroxide in pure water
Ka Ksp pH activity and lecture p17
How to Solve for Molar Solubility Given Ksp (All Work Shown) Practice Problems & Examples
Another Saturated Solution Equilibrium (Ksp) Sample Problem: Chapter 17 – Part 18
Introduction to solubility equilibria | Equilibrium | AP Chemistry | Khan Academy
Ksp: Equilibrium Constant for solubility Product
Комментарии
 0:41:52
0:41:52
 0:04:30
0:04:30
 0:04:52
0:04:52
 0:13:29
0:13:29
 0:01:10
0:01:10
 0:15:25
0:15:25
 0:14:49
0:14:49
 0:08:32
0:08:32
 0:19:14
0:19:14
 0:13:10
0:13:10
 0:06:39
0:06:39
 0:01:25
0:01:25
 0:05:23
0:05:23
 0:06:53
0:06:53
 0:05:48
0:05:48
 0:04:26
0:04:26
 0:03:17
0:03:17
 0:07:49
0:07:49
 0:04:55
0:04:55
 0:29:05
0:29:05
 0:05:52
0:05:52
 0:02:42
0:02:42
 0:08:17
0:08:17
 0:31:32
0:31:32