filmov
tv
Ionic Radius of Isoelectronic Species

Показать описание
Hi Learners,
This is the second video in the series of videos I will be uploading, regarding Periodic Classification.
In this video you will learn the simplest way to determine the ionic radius of isoelectronic species and arrange them in an order.
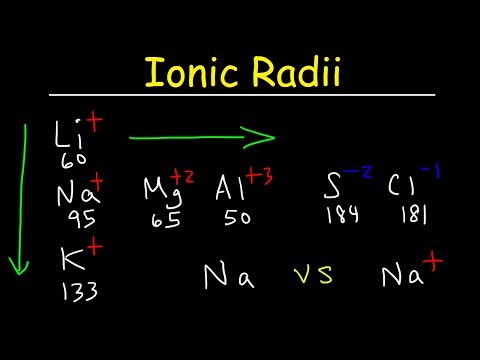
Isoelectronic species means atoms and ions having same number of electrons.
The question which I have discussed in this video is
Q) Consider the following species
N3-, O2-, F-, Na+, Mg2+, Al3+
a) What is common in them?
b) Arrange them in increasing ionic radii.
I am sure after watching this video you will be able to solve any random question related to determining ionic radius of Isoelectronic species.
If you have any doubt or you want me to cover any specific topic in chemistry please feel free to post a comment.
Within a day I will be uploading a video on same topic in Hindi.
Happy Learning :)
This is the second video in the series of videos I will be uploading, regarding Periodic Classification.
In this video you will learn the simplest way to determine the ionic radius of isoelectronic species and arrange them in an order.
Isoelectronic species means atoms and ions having same number of electrons.
The question which I have discussed in this video is
Q) Consider the following species
N3-, O2-, F-, Na+, Mg2+, Al3+
a) What is common in them?
b) Arrange them in increasing ionic radii.
I am sure after watching this video you will be able to solve any random question related to determining ionic radius of Isoelectronic species.
If you have any doubt or you want me to cover any specific topic in chemistry please feel free to post a comment.
Within a day I will be uploading a video on same topic in Hindi.
Happy Learning :)
Ionic Radius Trends, Basic Introduction, Periodic Table, Sizes of Isoelectric Ions, Chemistry
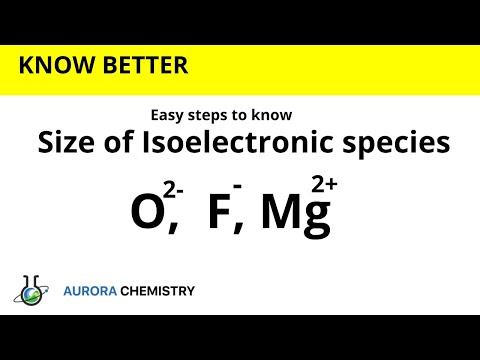
Size of isoelectronic species II Which is smaller in size? O2-, F-,Mg2+
Ionic Radius | Trends of Ionic Radius in Periodic Table
TRICK OF ISOELECTRONIC SPECIES 👉With NEET, JEE questions 👍
Isoelectronic Series Atomic Radius
Practice Problem: Atomic Radii and Ionic Radii
Trick to arrange size of Isoelectronic species🔥🔥. Chemistry. Class-11
Ionic Radii - Isoelectronic Species | Chem | Ashish Shekhar #neet2024 #shorts
What are Isoelectronic Species ??
AP Chemistry - Lesson 1.14 - Ionic Size and Isoelectronic Species
The increasing order of the ionic radii of the given isoelectronic species is :-
the increasing order of ionic radii of the given isoelectronic species is
Comparison of Ionic Radii of Isoelectronic Specs | Foundation Of Science
Radius of Isoelectronic Species - Classification of Elements | Class 11 Chemistry
Ionic Radius of Isoelectronic Species
IONIC RADIUS: ISOELECTRONIC SPECIES
Ionic Radius comparison question inorganic #chemistry #tricks #shorts
The increasing order of the ionic radii of the given isoelectronic species is :- | 12 | JEE MAIN...
3.2 Periodicity (Part D) - Variation of Radii in Isoelectronic Species
Class 11 Chemistry | Ionic radii and Isoelectronic species
7.3 Isoelectronic Species
The correct order of the decreasing ionic radii among the following isoelectronic species is:....
The increasing order of the ionic radii of the given isoelectronic species is: (a) Cl^-, Ca^2+, K...
Ionic radius and isoelectronic species in Tamil #class11cbse
Комментарии
 0:11:47
0:11:47
 0:02:29
0:02:29
 0:09:00
0:09:00
 0:05:21
0:05:21
 0:03:17
0:03:17
 0:07:27
0:07:27
 0:03:31
0:03:31
 0:00:44
0:00:44
 0:02:39
0:02:39
 0:06:58
0:06:58
 0:03:06
0:03:06
 0:01:53
0:01:53
 0:00:55
0:00:55
 0:17:42
0:17:42
 0:21:15
0:21:15
 0:08:46
0:08:46
 0:00:36
0:00:36
 0:05:34
0:05:34
 0:12:07
0:12:07
 0:19:46
0:19:46
 0:04:28
0:04:28
 0:03:37
0:03:37
 0:04:41
0:04:41
 0:11:54
0:11:54