filmov
tv
PV=nRT The Ideal Gas Law: What is it, What is R, four practice problems solved including molar mass

Показать описание
In addition to showing how to solve PV= nRT problems (see timings below), including those with mass and molar mass, this video explains how the ideal gas law, PV = nRT, was derived, and the various units and values of R, including how they are derived. There is also a brief explanation of what "ideal" refers to in the term "ideal gas." The timing of the four practice problems are:
1) solving for volume 4:00
2) solving for temperature 5:20
3) solving for molar mass (given mass of gas) 6:55
4) solving for mass (given molar mas of gas) 8:25
Check out other popular CC Academy videos on this channel:
Stoichiometry Tutorial, step by step
Types of Chemical Reactions: How to classify five basic reaction types
Solution Stoichiometry
Orbitals the Basics: Atomic Orbitals Tutorial
Hybrid Orbitals Explained
Polar Molecules Tutorial: How to determine polarity in a molecule
Metallic Bonding and Metallic Properties Explained
Covalent Bonding Tutorial
Ionic Bonds, Ionic Compounds: What is an ionic bond and how do ionic compounds form
Electronegativity and bond character (bond type): non-polar covalent, polar, ionic
Metric Unit Prefix Conversions: How to Convert Metric System Prefixes
Metric unit conversions shortcut: fast, easy how-to with examples
Mole Conversions Tutorial: how to convert mole - mass, mole - particle, mass - particle problems
Frequency, Wavelength, and the Speed of Light
The Bohr Model of the Atom and Atomic Emission Spectra
What is Heat: A brief introduction at the particle level
Rutherford's Gold Foil Experiment
Unit Conversion Using Dimensional Analysis Tutorial
What is Fire: Combustion Reaction Tutorial
Quantum Numbers Tutorial
Electron Configurations Tutorial and How to Derive Electron Configurations from the Periodic Table
Concentration and Molarity Explained
Heating Curves Tutorial
Naming Ionic Compounds
Limiting Reactant Tutorial
PV=nRT The Ideal Gas Law: What is it, What is R, Four practice problems solved including molar mass
Gas density and PV=nRT, the ideal gas law
Surface Tension - What is it, how does it form, what properties does it impart
Ideal Gas Law - Wikipedia - November 2020
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law.[1] The ideal gas law is often written in an empirical form:
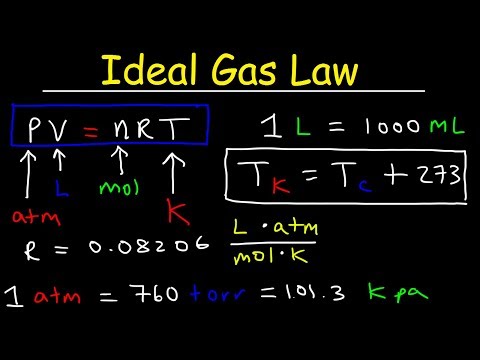
PV=nRT, where {\displaystyle P}P, {\displaystyle V}V and {\displaystyle T}T are the pressure, volume and temperature; {\displaystyle n}n is the amount of substance; and {\displaystyle R}R is the ideal gas constant. It is the same for all gases. It can also be derived from the microscopic kinetic theory, as was achieved (apparently independently) by August Krönig in 1856[2] and Rudolf Clausius in 1857.[3]
Note that this law makes no comment as to whether a gas heats or cools during compression or expansion. An ideal gas may not change temperature, but most gases like air are not ideal and follow the Joule–Thomson effect.
The state of an amount of gas is determined by its pressure, volume, and temperature. The modern form of the equation relates these simply in two main forms. The temperature used in the equation of state is an absolute temperature: the appropriate SI unit is the kelvin.[4]
Common forms
The most frequently introduced forms are:
pV=nRT=Nk
p is the pressure of the gas,
V is the volume of the gas,
n is the amount of substance of gas (also known as number of moles),
R is the ideal, or universal, gas constant, equal to the product of the Boltzmann constant and the Avogadro constant,
k is the Boltzmann constant
NA is the Avogadro constant
T is the absolute temperature of the gas.
In SI units, p is measured in pascals, V is measured in cubic metres, n is measured in moles, and T in kelvins (the Kelvin scale is a shifted Celsius scale, where 0.00 K = −273.15 °C, the lowest possible temperature). R has the value 8.314 J/(K·mol) ≈ 2 cal/(K·mol), or 0.0821 l·atm/(mol·K).
1) solving for volume 4:00
2) solving for temperature 5:20
3) solving for molar mass (given mass of gas) 6:55
4) solving for mass (given molar mas of gas) 8:25
Check out other popular CC Academy videos on this channel:
Stoichiometry Tutorial, step by step
Types of Chemical Reactions: How to classify five basic reaction types
Solution Stoichiometry
Orbitals the Basics: Atomic Orbitals Tutorial
Hybrid Orbitals Explained
Polar Molecules Tutorial: How to determine polarity in a molecule
Metallic Bonding and Metallic Properties Explained
Covalent Bonding Tutorial
Ionic Bonds, Ionic Compounds: What is an ionic bond and how do ionic compounds form
Electronegativity and bond character (bond type): non-polar covalent, polar, ionic
Metric Unit Prefix Conversions: How to Convert Metric System Prefixes
Metric unit conversions shortcut: fast, easy how-to with examples
Mole Conversions Tutorial: how to convert mole - mass, mole - particle, mass - particle problems
Frequency, Wavelength, and the Speed of Light
The Bohr Model of the Atom and Atomic Emission Spectra
What is Heat: A brief introduction at the particle level
Rutherford's Gold Foil Experiment
Unit Conversion Using Dimensional Analysis Tutorial
What is Fire: Combustion Reaction Tutorial
Quantum Numbers Tutorial
Electron Configurations Tutorial and How to Derive Electron Configurations from the Periodic Table
Concentration and Molarity Explained
Heating Curves Tutorial
Naming Ionic Compounds
Limiting Reactant Tutorial
PV=nRT The Ideal Gas Law: What is it, What is R, Four practice problems solved including molar mass
Gas density and PV=nRT, the ideal gas law
Surface Tension - What is it, how does it form, what properties does it impart
Ideal Gas Law - Wikipedia - November 2020
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law.[1] The ideal gas law is often written in an empirical form:
PV=nRT, where {\displaystyle P}P, {\displaystyle V}V and {\displaystyle T}T are the pressure, volume and temperature; {\displaystyle n}n is the amount of substance; and {\displaystyle R}R is the ideal gas constant. It is the same for all gases. It can also be derived from the microscopic kinetic theory, as was achieved (apparently independently) by August Krönig in 1856[2] and Rudolf Clausius in 1857.[3]
Note that this law makes no comment as to whether a gas heats or cools during compression or expansion. An ideal gas may not change temperature, but most gases like air are not ideal and follow the Joule–Thomson effect.
The state of an amount of gas is determined by its pressure, volume, and temperature. The modern form of the equation relates these simply in two main forms. The temperature used in the equation of state is an absolute temperature: the appropriate SI unit is the kelvin.[4]
Common forms
The most frequently introduced forms are:
pV=nRT=Nk
p is the pressure of the gas,
V is the volume of the gas,
n is the amount of substance of gas (also known as number of moles),
R is the ideal, or universal, gas constant, equal to the product of the Boltzmann constant and the Avogadro constant,
k is the Boltzmann constant
NA is the Avogadro constant
T is the absolute temperature of the gas.
In SI units, p is measured in pascals, V is measured in cubic metres, n is measured in moles, and T in kelvins (the Kelvin scale is a shifted Celsius scale, where 0.00 K = −273.15 °C, the lowest possible temperature). R has the value 8.314 J/(K·mol) ≈ 2 cal/(K·mol), or 0.0821 l·atm/(mol·K).
Комментарии
 0:03:40
0:03:40
 0:06:19
0:06:19
 0:05:18
0:05:18
 0:12:27
0:12:27
 0:01:59
0:01:59
 0:02:19
0:02:19
 0:23:31
0:23:31
 0:05:11
0:05:11
 0:02:44
0:02:44
 0:11:21
0:11:21
 0:01:00
0:01:00
 0:00:40
0:00:40
 0:06:59
0:06:59
 0:02:02
0:02:02
 0:19:24
0:19:24
 0:00:18
0:00:18
 0:04:18
0:04:18
 0:14:09
0:14:09
 0:03:20
0:03:20
 0:06:33
0:06:33
 0:02:55
0:02:55
 0:04:27
0:04:27
 0:04:25
0:04:25
 0:00:44
0:00:44