filmov
tv
Ideal Gas Law - PV=nRT - Straight Science

Показать описание
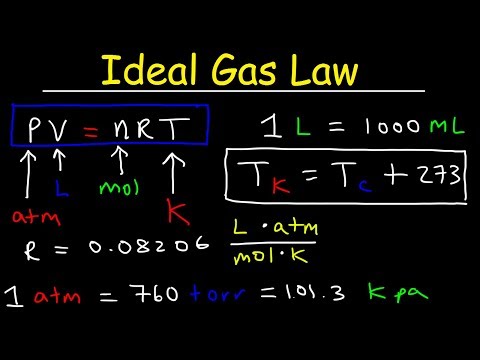
In this video we go over the ideal gas law: PV=nRT. There are no initial and final conditions in this one, instead, we look at how all four gas variables interact with one another.
We also look at the ideal gas constant: R = 0.0821 L atm mol^-1 K^-1.
I know those units look terrible, but they work out beautifully **IF** you make sure you have all your variables in the correct units! Pressure is in atmospheres (atm), volume is in liters (L), amount of moles is in moles... duh!... and temperature is in Kelvin (K).
Good luck. Hope everyone is doing well!
We also look at the ideal gas constant: R = 0.0821 L atm mol^-1 K^-1.
I know those units look terrible, but they work out beautifully **IF** you make sure you have all your variables in the correct units! Pressure is in atmospheres (atm), volume is in liters (L), amount of moles is in moles... duh!... and temperature is in Kelvin (K).
Good luck. Hope everyone is doing well!
The ideal gas law (PV = nRT) | Intermolecular forces and properties | AP Chemistry | Khan Academy
PV=nRT, the Ideal Gas Law, what is it and how to use it
What are the units for the Ideal Gas Law (PV=nRT)?
Ideal Gas Law Practice Problems
Ideal Gas Law (PV=nRT) Example Problem
Ideal Gas Law || PV=nRT
Ideal Gas Law - PV=nRT - Straight Science
The Ideal Gas Law: pV = nRT - IB Physics
Ideal Gas Law: PV = nRT
Ideal Gas Law (PV=nRT) Practice Problem
Ideal Gas Law PV nRT
The Ideal Gas Law, PV = nRT
The Ideal Gas Law: Crash Course Chemistry #12
Ideal Gas Law PV = nRT
Kinetic Molecular Theory and the Ideal Gas Laws
The Ideal Gas Law: PV=nRT
The ideal gas law (PV=nRT)
The Ideal Gas Law PV=nRT
The ideal gas law PV=nRT - simple example
Ideal Gas Law, PV=nRT
Ideal Gas Law
General Chemistry | Ideal Gas Law (PV=nRT) [Example #1]
The Ideal Gas Law - PV=nRT
Ideal Gas Law | General Gas Equation | Chemistry
Комментарии
 0:06:19
0:06:19
 0:03:40
0:03:40
 0:01:59
0:01:59
 0:12:27
0:12:27
 0:02:19
0:02:19
 0:09:42
0:09:42
 0:04:25
0:04:25
 0:23:31
0:23:31
 0:06:03
0:06:03
 0:02:55
0:02:55
 0:06:20
0:06:20
 0:29:08
0:29:08
 0:09:03
0:09:03
 0:03:23
0:03:23
 0:05:11
0:05:11
 0:22:05
0:22:05
 0:22:59
0:22:59
 0:14:03
0:14:03
 0:03:36
0:03:36
 0:31:10
0:31:10
 0:05:36
0:05:36
 0:06:33
0:06:33
 0:09:23
0:09:23
 0:06:59
0:06:59