filmov
tv
Soaps and Detergents | Learn with BYJU'S

Показать описание
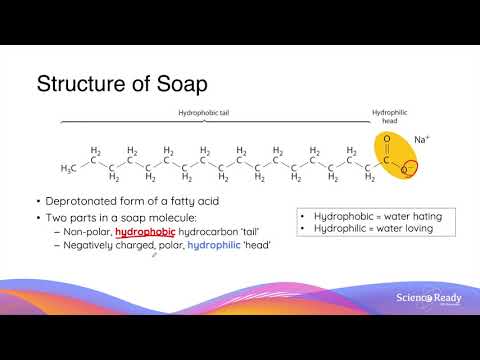
Soaps and Detergents are those substances which have the abilities to remove dirt from different surfaces when dissolved in water. A soap molecule consists of two ends which are the hydrophilic end and the hydrophobic end. The hydrophilic end has an affinity for water and attaches itself to the surrounding water. On the other hand, the hydrophobic end repels the water and attaches itself to the dirt.
The complete cleansing action of soaps and detergents is based on the hydrophobic end and the hydrophilic end. The soap or detergent molecules form a micelle in water and trap the water at the centre of the cluster. This dirt is contained in the micelle and remains suspended in the water in the form of a colloidal solution and can be easily washed away along with the dirt.
The complete cleansing action of soaps and detergents is based on the hydrophobic end and the hydrophilic end. The soap or detergent molecules form a micelle in water and trap the water at the centre of the cluster. This dirt is contained in the micelle and remains suspended in the water in the form of a colloidal solution and can be easily washed away along with the dirt.
 0:01:06
0:01:06
 0:02:56
0:02:56
 0:03:24
0:03:24
 0:02:38
0:02:38
 0:02:46
0:02:46
 0:02:15
0:02:15
 0:04:44
0:04:44
 0:01:53
0:01:53
 0:11:16
0:11:16
 0:07:02
0:07:02
 0:04:16
0:04:16
 0:01:00
0:01:00
 0:02:42
0:02:42
 0:03:08
0:03:08
 0:13:57
0:13:57
 0:00:46
0:00:46
 0:02:56
0:02:56
 0:04:34
0:04:34
![[5.1] Comparison between](https://i.ytimg.com/vi/YkYhpVHNcj4/hqdefault.jpg) 0:01:27
0:01:27
 0:00:29
0:00:29
 0:05:48
0:05:48
 0:00:30
0:00:30
 0:03:47
0:03:47
 0:11:09
0:11:09