filmov
tv
Transition Metals - Complex Shapes and Isomers|AQA A Level Chemistry

Показать описание
This video is intended to be an ultimate guide and revision on EVERY transition metal complex shape, bond angle and isomer you need to know for the 3.2.5.3 Shapes of complex ions section of the 3.2.5 Transition metals topic for the AQA A-Level chemistry specification. The aim of this video is to help all of you revise and prepare for your paper 1 and 3 exams.
👍 If you want to improve your grades and see more content like this - like, share, and subscribe to the channel! 📈
🕑In this video, we cover:
00:00 - What you will learn (AQA specification)
02:09 - Octahedral complexes with NH3 and H2O
07:45 - Octahedral complex cis-trans isomerism
14:49 - Octahedral complex bidentate ligands optical isomerism
27:27 - Tetrahedral complexes
29:48 - Square planar complex shape, isomerism, and cisplatin
33:54 - Linear complex
34:53 - Summary and spec check
35:57 - Skill development (specification)
37:34 - Practice questions (active recall)
📝 Resources 📖
- AQA specification:
📚 Although this tutorial focuses on AQA, the concepts covered here are also applicable to other exam boards, such as OCR and Edexcel
Комментарии
 0:48:40
0:48:40
 0:05:13
0:05:13
 0:13:42
0:13:42
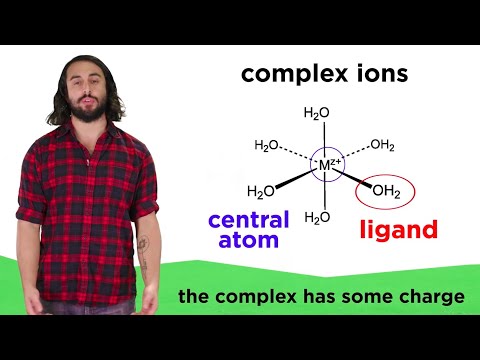 0:04:06
0:04:06
 0:03:07
0:03:07
 0:05:45
0:05:45
 0:11:59
0:11:59
 0:03:45
0:03:45
 0:12:05
0:12:05
 0:09:15
0:09:15
 0:29:43
0:29:43
 0:07:33
0:07:33
 0:11:21
0:11:21
 0:05:20
0:05:20
 0:01:36
0:01:36
 0:43:31
0:43:31
 0:00:51
0:00:51
 0:06:48
0:06:48
 0:14:44
0:14:44
 0:12:41
0:12:41
 0:42:47
0:42:47
 0:09:31
0:09:31
 0:12:15
0:12:15
 0:28:21
0:28:21