filmov
tv
Equilibrium constant expression for some important reactions || Chemistry with M faisal

Показать описание
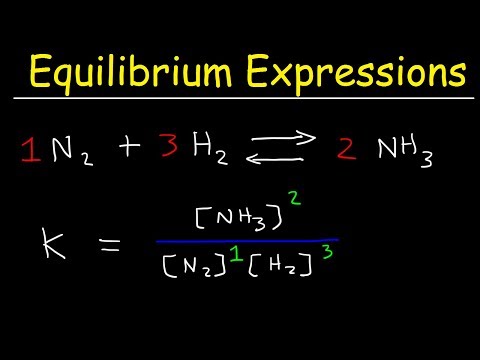
In this lecture discuss the equilibrium constent expression of some important reactions. Equilibrium constant expression for aqous and gasses for aqous and gasses phase. Equilibrium constent expression for formation of Ester, dissociation of PCl5, decomposition of N2O4, formation of NH3.
👉 Introduction of alcohol, phenol, either and Classification of alcohol
👉Nomenclature of alcohol and industrial preparation of methanol
Previous video
👉 Introduction of alcohol, phenol, either and Classification of alcohol
👉Physical properties of alcohol
👉 Preparation of phenol
👉Unit of equilibrium constent
#Chemistry_with_MFaisal
#Fsc_part_1_chemistry
#Chemistry_with_MFaisal
#chemical_equillibrium
#Law_of_mass_action
#equillibrium_constent
#lecture_about_Kc
#equillibrium_constent_expression
#formation_of_ester
#formation_of_ammonia
#decomposition_of_N2O4
#dissociation_of_PCl5
#faisal chemistry
My Whatsapp number
+92302-3585468
My email address is
-------------------------------------------------------------------------------------------------------------------------------
Note:
If you realise that this YouTube video helped you in chemistry, you can donate money to a video creator .This gesture shows motivation for video creator and an appreciation. The amount does not have to be big but it truly show the encouragement ,appreciation and support.
Muhammad Faisal Bilal
Meezan Bank-Depalpur, Sahiwal Area
Account Number: 98130103555528
Jazz Cash A/c no.
Ali Razaq
03023585468
👉 Introduction of alcohol, phenol, either and Classification of alcohol
👉Nomenclature of alcohol and industrial preparation of methanol
Previous video
👉 Introduction of alcohol, phenol, either and Classification of alcohol
👉Physical properties of alcohol
👉 Preparation of phenol
👉Unit of equilibrium constent
#Chemistry_with_MFaisal
#Fsc_part_1_chemistry
#Chemistry_with_MFaisal
#chemical_equillibrium
#Law_of_mass_action
#equillibrium_constent
#lecture_about_Kc
#equillibrium_constent_expression
#formation_of_ester
#formation_of_ammonia
#decomposition_of_N2O4
#dissociation_of_PCl5
#faisal chemistry
My Whatsapp number
+92302-3585468
My email address is
-------------------------------------------------------------------------------------------------------------------------------
Note:
If you realise that this YouTube video helped you in chemistry, you can donate money to a video creator .This gesture shows motivation for video creator and an appreciation. The amount does not have to be big but it truly show the encouragement ,appreciation and support.
Muhammad Faisal Bilal
Meezan Bank-Depalpur, Sahiwal Area
Account Number: 98130103555528
Jazz Cash A/c no.
Ali Razaq
03023585468
Комментарии
 0:05:24
0:05:24
 0:53:22
0:53:22
 0:06:15
0:06:15
 0:06:48
0:06:48
 0:04:32
0:04:32
 0:04:47
0:04:47
 0:07:03
0:07:03
 0:01:47
0:01:47
 0:17:53
0:17:53
 0:01:59
0:01:59
 0:10:20
0:10:20
![[Example] How to](https://i.ytimg.com/vi/0a9-j7ZumoQ/hqdefault.jpg) 0:01:35
0:01:35
 0:07:26
0:07:26
 0:15:03
0:15:03
 0:08:12
0:08:12
 0:03:00
0:03:00
 0:28:41
0:28:41
 0:12:43
0:12:43
 0:09:29
0:09:29
 0:26:40
0:26:40
 0:02:22
0:02:22
 0:00:15
0:00:15
 0:06:46
0:06:46
 0:02:37
0:02:37