filmov
tv
How to Write Equilibrium Constant Expression (K, Keq, Kc, Kp) Practice Problems, Examples, Summary

Показать описание
👉 Support me on Patreon 👈
💻 My highly recommended chemistry resources
HIGH SCHOOL / GENERAL CHEMISTRY
ORGANIC CHEMISTRY
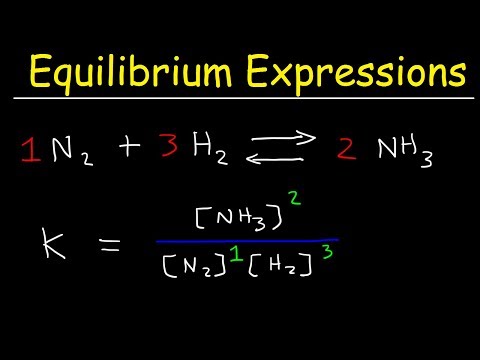
In this video, you'll learn how to write equilibrium constant expressions for any reactions. We'll go over what K, Keq, Kc, and Kp is and how to write the expression for them. Then we'll go over five different examples together
How To Write The Equilibrium Expression For a Chemical Reaction - Law of Mass Action
How to Write Equilibrium Constant Expression (K, Keq, Kc, Kp) Practice Problems, Examples, Summary
Writing Equilibrium Expressions
Chemical Equilibrium Constant K - Ice Tables - Kp and Kc
How to write the equilibrium expression (Kc): 3 Trick Questions
Chemical Equilibria and Reaction Quotients
The Equilibrium Constant
How to Write Equilibrium Constant Expression for Reactions for 2023 Jamb chemistry Class
Consider a horizontal plane parallel slab of gas of thickness that is maintained at a constant tempe
Writing equilibrium constant and reaction quotient expressions | AP Chemistry | Khan Academy
Keq Equilibrium Constant (EVERYTHING YOU NEED TO KNOW CHEMISTRY)
How to Write Equilibrium Expressions (Keq) 2017
How to Write Equilibrium Constant Expressions | Kc Keq Kp | Basics Chemical Equilibrium
Worked examples: Calculating equilibrium constants | Equilibrium | AP Chemistry | Khan Academy
Equilibrium Constants
ALEKS: Writing a concentration equilibrium constant expression
Using RICE to calculate equilibrium concentrations
Chem162 Writing Equilibrium Constant Expressions: Heterogeneous Equilibrium 14.2
Equilibrium Constant Grade 12: Exam
ALEKS - Writing an Equilibrium Constant for a Reaction Sequence
Equilibrium Constant (Example)
The Equilibrium Constant
Aleks Writing a pressure equilibrium constant expression
How to Write the Equilibrium Constant Expression for a Reaction (Chemical Equilibrium).
Комментарии
 0:05:24
0:05:24
 0:04:47
0:04:47
 0:10:20
0:10:20
 0:53:22
0:53:22
 0:04:32
0:04:32
 0:06:48
0:06:48
 0:06:15
0:06:15
 0:08:42
0:08:42
 0:04:38
0:04:38
 0:07:03
0:07:03
 0:09:06
0:09:06
 0:04:46
0:04:46
 0:07:31
0:07:31
 0:08:12
0:08:12
 0:01:47
0:01:47
 0:01:59
0:01:59
 0:10:13
0:10:13
 0:05:37
0:05:37
 0:06:48
0:06:48
 0:04:33
0:04:33
 0:10:28
0:10:28
 0:06:13
0:06:13
 0:02:37
0:02:37
 0:07:26
0:07:26