filmov
tv
Identifying Cis–Trans Isomers

Показать описание
Follow us:
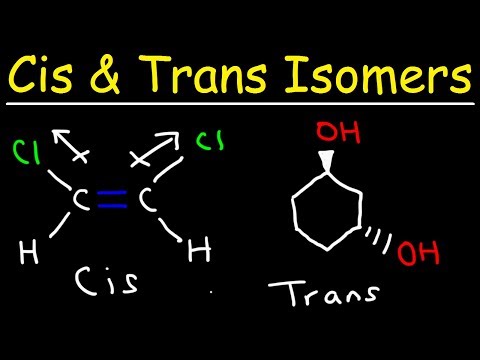
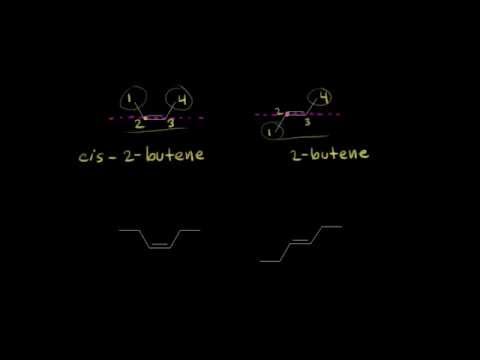
a. This is a cis isomer because the two halogen atoms attached to the carbon atoms of the double bond are on the same side. The name of the two-carbon alkene, starting with the bromo group on carbon 1, is cis-1-bromo-2-chloroethene.
b. This is a trans isomer because the two alkyl groups attached to the carbon atoms of the double bond are on opposite sides of the double bond. This isomer of the five-carbon alkene, 2-pentene, is named trans-2-pentene.
Which is preferred first in halogen naming - Cl or Br?
While writing, due to alphabetical preference, Bromo will be mentioned before Chloro, irrespective of their C number (position on longest chain), in IUPAC nomenclature.
While numbering, however, both Bromo & Chloro being substituents, do not enjoy any numbering priority, in case any major functional group is absent. Here we go by lowest sum rule. For e.g. - CH3CH(Cl)CH2CH(Br)CH2CH3 will be named as 4-Bromo-2-chlorohexane & not as 3-Bromo-5-chlorohexane.
While numbering, if from either side of the longest chain, both the substituents have same position, then lowest sum rule is redundant. In this case, both number wise and naming wise, Bromo is preferred over Chloro. For e.g. - CH3CH(Cl)CH2CH(Br)CH3 will be named as 2-Bromo-4-chloropentane & never as 4-Bromo-2-chloropentane.
Комментарии
 0:06:35
0:06:35
 0:05:24
0:05:24
 0:06:02
0:06:02
 0:02:37
0:02:37
 0:15:21
0:15:21
 0:10:27
0:10:27
 0:06:55
0:06:55
 0:03:35
0:03:35
 0:06:00
0:06:00
 0:09:14
0:09:14
 0:04:56
0:04:56
 0:10:01
0:10:01
 0:03:24
0:03:24
 0:11:15
0:11:15
 0:05:06
0:05:06
 0:02:58
0:02:58
 0:06:48
0:06:48
 0:03:19
0:03:19
 0:01:50
0:01:50
 0:02:28
0:02:28
 0:06:01
0:06:01
 0:09:57
0:09:57
 0:07:46
0:07:46
 0:05:27
0:05:27