filmov
tv
AQA 3.3 Halogenoalkanes REVISION

Показать описание
AQA 3.3 Halogenoalkanes REVISION
Halogenoalkanes | A-Level Chemistry | 3.3.3
Halogenoalkanes | A level Chemistry
Halogenoalkanes | A-level Chemistry | OCR, AQA, Edexcel
3.3.3 - Halogenoalkanes - Nucleophilic Substitution
Halogenoalkanes | A level Chemistry | Question Walkthrough
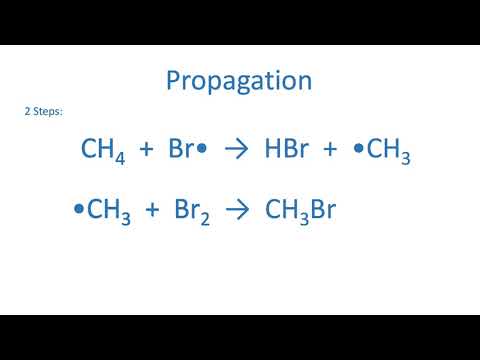
AQA A-Level Chemistry - Halogenoalkane synthesis (free radical substitution)
Y12 Halogenoalkanes (AQA A level Chemistry)
EVERY Organic Mechanism You NEED To Know|AQA A Level Chemistry Revision
Quick Revision - Haloalkanes
AQA 3.2 Alkanes REVISION
A Level Chemistry is EFFORTLESS Once You Learn This
3.3 Haloalkanes No 3 Nucleophilic substitution reactions - Ammonia and Water
AQA A level Chemistry - Forming halogenoalkanes by free radical substitution
Preparation of Halogenoalkane
Mechanisms | Halogenoalkanes | MCQ
Halogenoalkanes | A level Chemistry | MCQ Walkthrough
Nucleophilic Substitution of Halogenoalkanes with Hydroxide Ions, OH- (A-level Chemistry)
Nucleophilic Substitution Mechanisms - AQA A-Level Organic Chemistry
Elimination reactions of haloalkanes
AQA Chem - General Nucleophilic Substitution of Halogenoalkanes Reaction
TEST FOR HALOGENOALKANES [Last minute revision] | Chemistry at glance
Mechanisms | Explained | Year 12 or AS Chemistry | Organic Chemistry | A level Chemistry
Haloalkanes: Reactions and Mechanism | A-level Chemistry | OCR, AQA, Edexcel
Комментарии
 0:23:10
0:23:10
 0:31:44
0:31:44
 0:45:12
0:45:12
 0:11:03
0:11:03
 0:28:30
0:28:30
 0:15:08
0:15:08
 0:17:16
0:17:16
 0:43:48
0:43:48
 0:43:18
0:43:18
 0:05:49
0:05:49
 0:22:58
0:22:58
 0:05:30
0:05:30
 0:09:30
0:09:30
 0:09:25
0:09:25
 0:11:32
0:11:32
 0:00:55
0:00:55
 0:11:25
0:11:25
 0:10:13
0:10:13
 0:06:47
0:06:47
 0:11:04
0:11:04
 0:00:41
0:00:41
 0:05:41
0:05:41
 0:33:01
0:33:01
 0:03:22
0:03:22